IGF1 activates cell cycle arrest following irradiation by reducing binding of ΔNp63 to the p21 promoter
- PMID: 21480565
- PMCID: PMC2939491
- DOI: 10.1038/cddis.2010.28
IGF1 activates cell cycle arrest following irradiation by reducing binding of ΔNp63 to the p21 promoter
Abstract
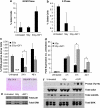
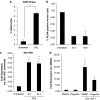
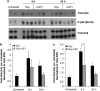
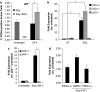
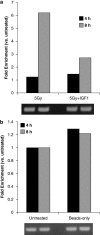
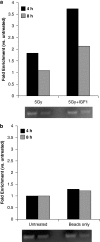
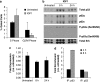
Radiotherapy for head and neck tumors often results in persistent loss of function in salivary glands. Patients suffering from impaired salivary function frequently terminate treatment prematurely because of reduced quality of life caused by malnutrition and other debilitating side-effects. It has been previously shown in mice expressing a constitutively active form of Akt (myr-Akt1), or in mice pretreated with IGF1, apoptosis is suppressed, which correlates with maintained salivary gland function measured by stimulated salivary flow. Induction of cell cycle arrest may be important for this protection by allowing cells time for DNA repair. We have observed increased accumulation of cells in G2/M at acute time-points after irradiation in parotid glands of mice receiving pretreatment with IGF1. As p21, a transcriptional target of the p53 family, is necessary for maintaining G2/M arrest, we analyzed the roles of p53 and p63 in modulating IGF1-stimulated p21 expression. Pretreatment with IGF1 reduces binding of ΔNp63 to the p21 promoter after irradiation, which coincides with increased p53 binding and sustained p21 transcription. Our data indicate a role for ΔNp63 in modulating p53-dependent gene expression and influencing whether a cell death or cell cycle arrest program is initiated.
Keywords: IGF1; cell cycle arrest; p53; p63; radiation; xerostomia.
Figures
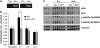
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. - PubMed
-
- Eisbruch A, Ten Haken RK, Kim HM, Marsh LH, Ship JA. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 1999;45:577–587. - PubMed
-
- Banin S, Moyal L, Shieh S, Taya Y, Anderson CW, Chessa L, et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–1677. - PubMed
-
- Canman CE, Lim DS, Cimprich KA, Taya Y, Tamai K, Sakaguchi K, et al. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–1679. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

