Golgi glycosylation
- PMID: 21441588
- PMCID: PMC3062213
- DOI: 10.1101/cshperspect.a005199
Golgi glycosylation
Abstract
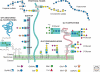
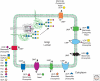
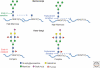
Glycosylation is a very common modification of protein and lipid, and most glycosylation reactions occur in the Golgi. Although the transfer of initial sugar(s) to glycoproteins or glycolipids occurs in the ER or on the ER membrane, the subsequent addition of the many different sugars that make up a mature glycan is accomplished in the Golgi. Golgi membranes are studded with glycosyltransferases, glycosidases, and nucleotide sugar transporters arrayed in a generally ordered manner from the cis-Golgi to the trans-Golgi network (TGN), such that each activity is able to act on specific substrate(s) generated earlier in the pathway. The spectrum of glycosyltransferases and other activities that effect glycosylation may vary with cell type, and thus the final complement of glycans on glycoconjugates is variable. In addition, glycan synthesis is affected by Golgi pH, the integrity of Golgi peripheral membrane proteins, growth factor signaling, Golgi membrane dynamics, and cellular stress. Knowledge of Golgi glycosylation has fostered the development of assays to identify mechanisms of intracellular vesicular trafficking and facilitated glycosylation engineering of recombinant glycoproteins.
Figures

Similar articles
-
Glycosylation Quality Control by the Golgi Structure.J Mol Biol. 2016 Aug 14;428(16):3183-3193. doi: 10.1016/j.jmb.2016.02.030. Epub 2016 Mar 5. J Mol Biol. 2016. PMID: 26956395 Free PMC article. Review.
-
Glycosylation disorders of membrane trafficking.Glycoconj J. 2013 Jan;30(1):23-31. doi: 10.1007/s10719-012-9389-y. Epub 2012 May 15. Glycoconj J. 2013. PMID: 22584409 Review.
-
Cisterna-specific localization of glycosylation-related proteins to the Golgi apparatus.Cell Struct Funct. 2012;37(1):55-63. doi: 10.1247/csf.11037. Epub 2012 Jan 17. Cell Struct Funct. 2012. PMID: 22251795
-
What can yeast tell us about N-linked glycosylation in the Golgi apparatus?FEBS Lett. 2001 Jun 8;498(2-3):223-7. doi: 10.1016/s0014-5793(01)02488-7. FEBS Lett. 2001. PMID: 11412862 Review.
-
Conserved oligomeric Golgi complex specifically regulates the maintenance of Golgi glycosylation machinery.Glycobiology. 2011 Dec;21(12):1554-69. doi: 10.1093/glycob/cwr028. Epub 2011 Mar 18. Glycobiology. 2011. PMID: 21421995 Free PMC article.
Cited by
-
Immune Epitopes of SARS-CoV-2 Spike Protein and Considerations for Universal Vaccine Development.bioRxiv [Preprint]. 2023 Oct 27:2023.10.26.564184. doi: 10.1101/2023.10.26.564184. bioRxiv. 2023. Update in: Immunohorizons. 2024 Mar 1;8(3):214-226. doi: 10.4049/immunohorizons.2400003 PMID: 37961687 Free PMC article. Updated. Preprint.
-
Loss of N-acetylgalactosaminyltransferase 3 in poorly differentiated pancreatic cancer: augmented aggressiveness and aberrant ErbB family glycosylation.Br J Cancer. 2016 Jun 14;114(12):1376-86. doi: 10.1038/bjc.2016.116. Epub 2016 May 17. Br J Cancer. 2016. PMID: 27187683 Free PMC article.
-
Autoantibodies in the pathogenesis of idiopathic inflammatory myopathies: Does the endoplasmic reticulum stress response have a role?Front Immunol. 2022 Sep 15;13:940122. doi: 10.3389/fimmu.2022.940122. eCollection 2022. Front Immunol. 2022. PMID: 36189221 Free PMC article. Review.
-
Acute GARP depletion disrupts vesicle transport, leading to severe defects in sorting, secretion, and O-glycosylation.bioRxiv [Preprint]. 2024 Oct 14:2024.10.07.617053. doi: 10.1101/2024.10.07.617053. bioRxiv. 2024. PMID: 39416116 Free PMC article. Preprint.
-
The Methods Employed in Mass Spectrometric Analysis of Posttranslational Modifications (PTMs) and Protein-Protein Interactions (PPIs).Adv Exp Med Biol. 2019;1140:169-198. doi: 10.1007/978-3-030-15950-4_10. Adv Exp Med Biol. 2019. PMID: 31347048 Free PMC article. Review.
References
-
- Aikawa J, Grobe K, Tsujimoto M, Esko JD 2001. Multiple isozymes of heparan sulfate/heparin GlcNAc N-deacetylase/GlcN N-sulfotransferase. Structure and activity of the fourth member, NDST4. J Biol Chem 276: 5876–5882 - PubMed
-
- Aoki K, Tiemeyer M 2010. The glycomics of glycan glucuronylation in Drosophila melanogaster. Methods Enzymol 480: 297–321 - PubMed
-
- Aoki K, Perlman M, Lim JM, Cantu R, Wells L, Tiemeyer M 2007. Dynamic developmental elaboration of N-linked glycan complexity in the Drosophila melanogaster embryo. J Biol Chem 282: 9127–9142 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
