Exophilin8 transiently clusters insulin granules at the actin-rich cell cortex prior to exocytosis
- PMID: 21441305
- PMCID: PMC3093323
- DOI: 10.1091/mbc.E10-05-0404
Exophilin8 transiently clusters insulin granules at the actin-rich cell cortex prior to exocytosis
Abstract
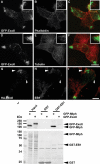
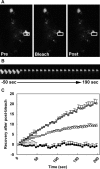
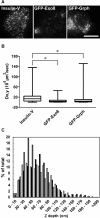
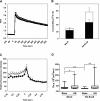
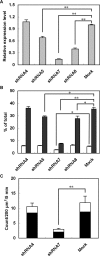
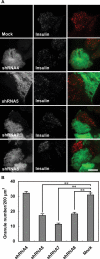
Exophilin8/MyRIP/Slac2-c is an effector protein of the small GTPase Rab27a and is specifically localized on retinal melanosomes and secretory granules. We investigated the role of exophilin8 in insulin granule trafficking. Exogenous expression of exophilin8 in pancreatic β cells or their cell line, MIN6, polarized (exophilin8-positive) insulin granules at the cell corners, where both cortical actin and the microtubule plus-end-binding protein, EB1, were present. Mutation analyses indicated that the ability of exophilin8 to act as a linker between Rab27a and myosin Va is essential for its granule-clustering activity. Moreover, exophilin8 and exophilin8-associated insulin granules were markedly stable and immobile. Total internal reflection fluorescence microscopy indicated that exophilin8 restricts the motion of insulin granules at a region deeper than that where another Rab27a effector, granuphilin, accumulates docked granules directly attached to the plasma membrane. However, the exophilin8-induced immobility of insulin granules was eliminated upon secretagogue stimulation and did not inhibit evoked exocytosis. Furthermore, exophilin8 depletion prevents insulin granules from being transported close to the plasma membrane and inhibits their fusion. These findings indicate that exophilin8 transiently traps insulin granules into the cortical actin network close to the microtubule plus-ends and supplies them for release during the stimulation.
Figures


Similar articles
-
The Rab27a effector exophilin7 promotes fusion of secretory granules that have not been docked to the plasma membrane.Mol Biol Cell. 2013 Feb;24(3):319-30. doi: 10.1091/mbc.E12-04-0265. Epub 2012 Dec 5. Mol Biol Cell. 2013. PMID: 23223571 Free PMC article.
-
Involvement of the Rab27 binding protein Slac2c/MyRIP in insulin exocytosis.Mol Biol Cell. 2003 Oct;14(10):4103-13. doi: 10.1091/mbc.e03-01-0022. Epub 2003 Aug 7. Mol Biol Cell. 2003. PMID: 14517322 Free PMC article.
-
Docking is not a prerequisite but a temporal constraint for fusion of secretory granules.Traffic. 2008 Jul;9(7):1191-203. doi: 10.1111/j.1600-0854.2008.00744.x. Epub 2008 Apr 4. Traffic. 2008. PMID: 18397364
-
Multiple pathways and independent functional pools in insulin granule exocytosis.Genes Cells. 2023 Jul;28(7):471-481. doi: 10.1111/gtc.13029. Epub 2023 Apr 18. Genes Cells. 2023. PMID: 37070774 Free PMC article. Review.
-
Rab27a, actin and beta-cell endocytosis.Endocr J. 2011;58(1):1-6. doi: 10.1507/endocrj.k10e-391. Epub 2010 Dec 23. Endocr J. 2011. PMID: 21187662 Review.
Cited by
-
The circular RNA Cdr1as, via miR-7 and its targets, regulates insulin transcription and secretion in islet cells.Sci Rep. 2015 Jul 27;5:12453. doi: 10.1038/srep12453. Sci Rep. 2015. PMID: 26211738 Free PMC article.
-
Myrip couples the capture of secretory granules by the actin-rich cell cortex and their attachment to the plasma membrane.J Neurosci. 2012 Feb 15;32(7):2564-77. doi: 10.1523/JNEUROSCI.2724-11.2012. J Neurosci. 2012. PMID: 22396429 Free PMC article.
-
Coronin1C Is a GDP-Specific Rab44 Effector That Controls Osteoclast Formation by Regulating Cell Motility in Macrophages.Int J Mol Sci. 2022 Jun 14;23(12):6619. doi: 10.3390/ijms23126619. Int J Mol Sci. 2022. PMID: 35743062 Free PMC article.
-
Granuphilin exclusively mediates functional granule docking to the plasma membrane.Sci Rep. 2016 Apr 1;6:23909. doi: 10.1038/srep23909. Sci Rep. 2016. PMID: 27032672 Free PMC article.
-
MyRIP interaction with MyoVa on secretory granules is controlled by the cAMP-PKA pathway.Mol Biol Cell. 2012 Nov;23(22):4444-55. doi: 10.1091/mbc.E12-05-0369. Epub 2012 Sep 19. Mol Biol Cell. 2012. PMID: 22993210 Free PMC article.
References
-
- Fukuda M, Kuroda TS. Slac2-c (synaptotagmin-like protein homologue lacking C2 domains-c), a novel linker protein that interacts with Rab27, myosin Va/VIIa, and actin. J Biol Chem. 2002;277:43096–43103. - PubMed
-
- Fukuda M, Kuroda TS, Mikoshiba K. Slac2-a/melanophilin, the missing link between Rab27 and myosin Va: implications of a tripartite protein complex for melanosome transport. J Biol Chem. 2002;277:12432–12436. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

