The golgin coiled-coil proteins of the Golgi apparatus
- PMID: 21436057
- PMCID: PMC3098672
- DOI: 10.1101/cshperspect.a005256
The golgin coiled-coil proteins of the Golgi apparatus
Abstract
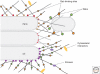
A number of long coiled-coil proteins are present on the Golgi. Often referred to as "golgins," they are well conserved in evolution and at least five are likely to have been present in the last common ancestor of all eukaryotes. Individual golgins are found in different parts of the Golgi stack, and they are typically anchored to the membrane at their carboxyl termini by a transmembrane domain or by binding a small GTPase. They appear to have roles in membrane traffic and Golgi structure, but their precise function is in most cases unclear. Many have binding sites for Rab family GTPases along their length, and this has led to the suggestion that the golgins act collectively to form a tentacular matrix that surrounds the Golgi to capture Rab-coated membranes in the vicinity of the stack. Such a collective role might explain the lack of cell lethality seen following loss of some of the genes in human familial conditions or mouse models.
Figures

Similar articles
-
The golgin coiled-coil proteins capture different types of transport carriers via distinct N-terminal motifs.BMC Biol. 2017 Jan 26;15(1):3. doi: 10.1186/s12915-016-0345-3. BMC Biol. 2017. PMID: 28122620 Free PMC article.
-
A novel Rab6-interacting domain defines a family of Golgi-targeted coiled-coil proteins.Curr Biol. 1999 Apr 8;9(7):381-4. doi: 10.1016/s0960-9822(99)80167-5. Curr Biol. 1999. PMID: 10209123
-
Golgins in the structure and dynamics of the Golgi apparatus.Curr Opin Cell Biol. 2003 Aug;15(4):405-13. doi: 10.1016/s0955-0674(03)00054-1. Curr Opin Cell Biol. 2003. PMID: 12892780 Review.
-
Golgins and GTPases, giving identity and structure to the Golgi apparatus.Biochim Biophys Acta. 2005 Jul 10;1744(3):383-95. doi: 10.1016/j.bbamcr.2005.02.001. Epub 2005 Feb 25. Biochim Biophys Acta. 2005. PMID: 15979508 Review.
-
Golgi coiled-coil proteins contain multiple binding sites for Rab family G proteins.J Cell Biol. 2008 Nov 17;183(4):607-15. doi: 10.1083/jcb.200808018. Epub 2008 Nov 10. J Cell Biol. 2008. PMID: 19001129 Free PMC article.
Cited by
-
Action of Arl1 GTPase and golgin Imh1 in Ypt6-independent retrograde transport from endosomes to the trans-Golgi network.Mol Biol Cell. 2019 Apr 1;30(8):1008-1019. doi: 10.1091/mbc.E18-09-0579. Epub 2019 Feb 6. Mol Biol Cell. 2019. PMID: 30726160 Free PMC article.
-
The SNARE-associated protein Sft2 functions in Imh1-mediated SNARE recycling transport upon ER stress.Mol Biol Cell. 2023 Oct 1;34(11):ar112. doi: 10.1091/mbc.E23-01-0019. Epub 2023 Aug 23. Mol Biol Cell. 2023. PMID: 37610835 Free PMC article.
-
The Golgin protein Coy1 functions in intra-Golgi retrograde transport and interacts with the COG complex and Golgi SNAREs.Mol Biol Cell. 2017 Aug 9;28(20):2686-700. doi: 10.1091/mbc.E17-03-0137. Online ahead of print. Mol Biol Cell. 2017. PMID: 28794270 Free PMC article.
-
The golgin coiled-coil proteins capture different types of transport carriers via distinct N-terminal motifs.BMC Biol. 2017 Jan 26;15(1):3. doi: 10.1186/s12915-016-0345-3. BMC Biol. 2017. PMID: 28122620 Free PMC article.
-
TBC1D23 is a bridging factor for endosomal vesicle capture by golgins at the trans-Golgi.Nat Cell Biol. 2017 Dec;19(12):1424-1432. doi: 10.1038/ncb3627. Epub 2017 Oct 30. Nat Cell Biol. 2017. PMID: 29084197 Free PMC article.
References
-
- Banu Y, Matsuda M, Yoshihara M, Kondo M, Sutou S, Matsukuma S 2002. Golgi matrix protein gene, Golga3/Mea2, rearranged and re-expressed in pachytene spermatocytes restores spermatogenesis in the mouse. Mol Reprod Dev 61: 288–301 - PubMed
-
- Barr FA 1999. A novel Rab6-interacting domain defines a family of Golgi-targeted coiled-coil proteins. Curr Biol 9: 381–384 - PubMed
-
- Barr FA, Puype M, Vandekerckhove J, Warren G 1997. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell 91: 253–262 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
