Sleep disturbances in highly stress reactive mice: modeling endophenotypes of major depression
- PMID: 21435199
- PMCID: PMC3068984
- DOI: 10.1186/1471-2202-12-29
Sleep disturbances in highly stress reactive mice: modeling endophenotypes of major depression
Abstract
Background: Neuronal mechanisms underlying affective disorders such as major depression (MD) are still poorly understood. By selectively breeding mice for high (HR), intermediate (IR), or low (LR) reactivity of the hypothalamic-pituitary-adrenocortical (HPA) axis, we recently established a new genetic animal model of extremes in stress reactivity (SR). Studies characterizing this SR mouse model on the behavioral, endocrine, and neurobiological levels revealed several similarities with key endophenotypes observed in MD patients. HR mice were shown to have changes in rhythmicity and sleep measures such as rapid eye movement sleep (REMS) and non-REM sleep (NREMS) as well as in slow wave activity, indicative of reduced sleep efficacy and increased REMS. In the present study we were interested in how far a detailed spectral analysis of several electroencephalogram (EEG) parameters, including relevant frequency bands, could reveal further alterations of sleep architecture in this animal model. Eight adult males of each of the three breeding lines were equipped with epidural EEG and intramuscular electromyogram (EMG) electrodes. After recovery, EEG and EMG recordings were performed for two days.
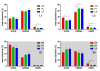
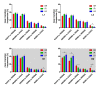
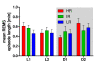
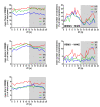
Results: Differences in the amount of REMS and wakefulness and in the number of transitions between vigilance states were found in HR mice, when compared with IR and LR animals. Increased frequencies of transitions from NREMS to REMS and from REMS to wakefulness in HR animals were robust across the light-dark cycle. Detailed statistical analyses of spectral EEG parameters showed that especially during NREMS the power of the theta (6-9 Hz), alpha (10-15 Hz) and eta (16-22.75 Hz) bands was significantly different between the three breeding lines. Well defined distributions of significant power differences could be assigned to different times during the light and the dark phase. Especially during NREMS, group differences were robust and could be continuously monitored across the light-dark cycle.
Conclusions: The HR mice, i.e. those animals that have a genetic predisposition to hyper-activating their HPA axis in response to stressors, showed disturbed patterns in sleep architecture, similar to what is known from depressed patients. Significant alterations in several frequency bands of the EEG, which also seem to at least partly mimic clinical observations, suggest the SR mouse lines as a promising animal model for basic research of mechanisms underlying sleep impairments in MD.
Figures

Similar articles
-
Rhythmicity in mice selected for extremes in stress reactivity: behavioural, endocrine and sleep changes resembling endophenotypes of major depression.PLoS One. 2009;4(1):e4325. doi: 10.1371/journal.pone.0004325. Epub 2009 Jan 29. PLoS One. 2009. PMID: 19177162 Free PMC article.
-
Mice selected for high versus low stress reactivity: a new animal model for affective disorders.Psychoneuroendocrinology. 2008 Jul;33(6):839-62. doi: 10.1016/j.psyneuen.2008.03.013. Epub 2008 May 23. Psychoneuroendocrinology. 2008. PMID: 18502051
-
Mice selected for extremes in stress reactivity reveal key endophenotypes of major depression: a translational approach.Psychoneuroendocrinology. 2014 Nov;49:229-43. doi: 10.1016/j.psyneuen.2014.07.008. Epub 2014 Jul 23. Psychoneuroendocrinology. 2014. PMID: 25123105
-
Counterpointing the functional role of the forebrain and of the brainstem in the control of the sleep-waking system.J Sleep Res. 2004 Sep;13(3):179-208. doi: 10.1111/j.1365-2869.2004.00412.x. J Sleep Res. 2004. PMID: 15339255 Review.
-
Cortical and subcortical EEG in relation to sleep-wake behavior in mammalian species.Neuropsychobiology. 1993;28(3):154-9. doi: 10.1159/000119017. Neuropsychobiology. 1993. PMID: 8278030 Review.
Cited by
-
Animal models of stress vulnerability and resilience in translational research.Curr Psychiatry Rep. 2012 Apr;14(2):159-65. doi: 10.1007/s11920-012-0256-0. Curr Psychiatry Rep. 2012. PMID: 22278810 Review.
-
Out Like a Light? The Effects of a Diurnal Husbandry Schedule on Mouse Sleep and Behavior.J Am Assoc Lab Anim Sci. 2018 Mar 1;57(2):124-133. J Am Assoc Lab Anim Sci. 2018. PMID: 29555001 Free PMC article.
-
Distinct Parameters in the EEG of the PLP α-SYN Mouse Model for Multiple System Atrophy Reinforce Face Validity.Front Behav Neurosci. 2017 Jan 10;10:252. doi: 10.3389/fnbeh.2016.00252. eCollection 2016. Front Behav Neurosci. 2017. PMID: 28119583 Free PMC article.
-
Discriminating rapid eye movement sleep from wakefulness by analyzing high frequencies from single-channel EEG recordings in mice.Sci Rep. 2023 Jun 13;13(1):9608. doi: 10.1038/s41598-023-36520-7. Sci Rep. 2023. PMID: 37311847 Free PMC article.
-
Early and later life stress alter brain activity and sleep in rats.PLoS One. 2013 Jul 26;8(7):e69923. doi: 10.1371/journal.pone.0069923. Print 2013. PLoS One. 2013. PMID: 23922857 Free PMC article.
References
-
- Von Holst D. The Concept of Stress and Its Relevance for Animal Behavior. Adv Study Behav. 1998;27:1–131. full_text.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

