Implantation of sinoatrial node cells into canine right ventricle: biological pacing appears limited by the substrate
- PMID: 21429290
- PMCID: PMC3692269
- DOI: 10.3727/096368911X565038
Implantation of sinoatrial node cells into canine right ventricle: biological pacing appears limited by the substrate
Abstract
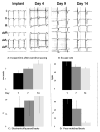
Biological pacing has been proposed as a physiologic counterpart to electronic pacing, and the sinoatrial node (SAN) is the general standard for biological pacemakers. We tested the expression of SAN pacemaker cell activity when implanted autologously in the right ventricle (RV). We induced complete heart block and implanted electronic pacemakers in the RV of adult mongrel dogs. Autologous SAN cells isolated enzymatically were studied by patch clamp to confirm SAN identity. SAN cells (400,000) were injected into the RV subepicardial free wall and dogs were monitored for 2 weeks. Pacemaker function was assessed by overdrive pacing and IV epinephrine challenge. SAN cells expressed a time-dependent inward current (I(f)) activating on hyperpolarization: density = 4.3 ± 0.6 pA/pF at -105 mV. Four of the six dogs demonstrated >50% of beats originating from the implant site at 24 h. Biological pacemaker rates on days 7-14 = 45-55 bpm and post-overdrive escape times = 1.5-2.5 s. Brisk catecholamine responsiveness occurred. Dogs implanted with autologous SAN cells manifest biological pacing properties dissimilar from those of the anatomic SAN. This highlights the importance of cell and substrate interaction in generating biological pacemaker function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Comparative effects of overdrive on sinus and subsidiary pacemaker function.Am Heart J. 1977 Mar;93(3):367-74. doi: 10.1016/s0002-8703(77)80256-1. Am Heart J. 1977. PMID: 65911
-
[Transplantation of pedicled autologous sinoatrial node tissue for treatment of complete atrioventricular block in dogs].Nan Fang Yi Ke Da Xue Xue Bao. 2013 Oct;33(10):1517-20. Nan Fang Yi Ke Da Xue Xue Bao. 2013. PMID: 24144759 Chinese.
-
Contribution of small conductance K+ channels to sinoatrial node pacemaker activity: insights from atrial-specific Na+ /Ca2+ exchange knockout mice.J Physiol. 2017 Jun 15;595(12):3847-3865. doi: 10.1113/JP274249. Epub 2017 May 13. J Physiol. 2017. PMID: 28346695 Free PMC article.
-
Pacing the Heart with Genes: Recent Progress in Biological Pacing.Curr Cardiol Rep. 2015 Aug;17(8):65. doi: 10.1007/s11886-015-0620-x. Curr Cardiol Rep. 2015. PMID: 26116393 Free PMC article. Review.
-
Pacemaker activity of the human sinoatrial node: effects of HCN4 mutations on the hyperpolarization-activated current.Europace. 2014 Mar;16(3):384-95. doi: 10.1093/europace/eut348. Europace. 2014. PMID: 24569893 Review.
Cited by
-
Overexpression of Map3k7 activates sinoatrial node-like differentiation in mouse ES-derived cardiomyocytes.PLoS One. 2017 Dec 27;12(12):e0189818. doi: 10.1371/journal.pone.0189818. eCollection 2017. PLoS One. 2017. PMID: 29281682 Free PMC article.
-
Transcription factor Tbx18 induces the differentiation of c-kit+ canine mesenchymal stem cells (cMSCs) into SAN-like pacemaker cells in a co-culture model in vitro.Am J Transl Res. 2018 Aug 15;10(8):2511-2528. eCollection 2018. Am J Transl Res. 2018. PMID: 30210689 Free PMC article.
-
Role of sinoatrial node architecture in maintaining a balanced source-sink relationship and synchronous cardiac pacemaking.Front Physiol. 2014 Nov 26;5:446. doi: 10.3389/fphys.2014.00446. eCollection 2014. Front Physiol. 2014. PMID: 25505419 Free PMC article. Review.
-
Human sinoatrial node structure: 3D microanatomy of sinoatrial conduction pathways.Prog Biophys Mol Biol. 2016 Jan;120(1-3):164-78. doi: 10.1016/j.pbiomolbio.2015.12.011. Epub 2015 Dec 30. Prog Biophys Mol Biol. 2016. PMID: 26743207 Free PMC article.
-
Cellular and Molecular Mechanisms of Functional Hierarchy of Pacemaker Clusters in the Sinoatrial Node: New Insights into Sick Sinus Syndrome.J Cardiovasc Dev Dis. 2021 Apr 13;8(4):43. doi: 10.3390/jcdd8040043. J Cardiovasc Dev Dis. 2021. PMID: 33924321 Free PMC article. Review.
References
-
- Anderson R, Ho S. The architecture of the sinus node, the atrioventricular conduction axis and the intermodal atrial myocardium. J Cardiovasc Electrophysiol. 1998;9:1233–1248. - PubMed
-
- Barbuti A, DiFrancesco D. Control of cardiac rate by “funny” channels in health and disease. Ann NY Acad Sci. 2008;1123:213–223. - PubMed
-
- Bogdanov KY, Maltsev VA, Vinogradova TM, Lyashkov AE, Spurgeon HA, Stern MD, Lakatta EG. Membrane potential fluctuations resulting from sub-membrane Ca2+ releases in rabbit sinoatrial nodal cells impart an exponential phase to the late diastolic depolarization that controls their chronotropic state. Circ Res. 2006;99:979–987. - PubMed
-
- Brooks CMcC, Lu HH. The sinoatrial pacemaker of the heart. Fort Lauderdale, FL: Charles C. Thomas; 1972.
-
- Bucchi A, Plotnikov AN, Shlapakova I, Danilo P, Jr, Kryukova Y, Qu J, Lu Z, Liu H, Pan Z, Potapova I, KenKnight B, Girouard S, Cohen IS, Brink PR, Robinson RB, Rosen MR. Wild-type and mutant HCN channels in a tandem biological-electronic cardiac pacemaker. Circulation. 2006;114:992–999. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

