RCMV increases intimal hyperplasia by inducing inflammation, MCP-1 expression and recruitment of adventitial cells to intima
- PMID: 21429242
- PMCID: PMC3063229
- DOI: 10.1186/2042-4280-1-7
RCMV increases intimal hyperplasia by inducing inflammation, MCP-1 expression and recruitment of adventitial cells to intima
Abstract
Background: Cytomegalovirus (CMV) infection has been associated with accelerated transplant vasculopathy. In this study, we assessed the effects of acute rat CMV (RCMV) infection on vessel remodeling in transplant vasculopathy, focusing on allograft morphology, inflammation and contribution of adventitial cells to intimal hyperplasia.
Methods: Infrarenal aorta was locally infected with RCMV and transplanted from female F344 rats to male Lewis rats. Graft samples were collected 2 and 8 weeks after transplantation and analyzed for intimal hyperplasia, collagen degradation and inflammation. Transplantation of aorta followed by transplantation of RCMV infected and labeled isogenic adventitia were performed to study migration of adventitial cells towards the intima.
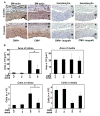
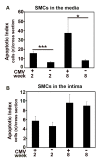
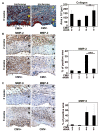
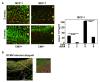
Results: Intimal hyperplasia was increased threefold in infected allografts. RCMV induced apoptosis in the media, expression of matrix metalloproteinase 2, and decreased collagen deposits. Macrophage infiltration was increased in the infected allografts and resulted in increased production of MCP-1. RCMV-infected macrophages were observed in the adventitia and intima. Cells derived from infected adventitia migrated towards the intima of the allograft.
Conclusions: RCMV enhances infiltration of macrophages to the allografts, and thereby increases MCP-1 production and inflammation, followed by recruitment of adventitial cells to the intima and accelerated intimal hyperplasia.
Figures

Similar articles
-
Cytomegalovirus antigen expression, endothelial cell proliferation, and intimal thickening in rat cardiac allografts after cytomegalovirus infection.Circulation. 1995 Nov 1;92(9):2594-604. doi: 10.1161/01.cir.92.9.2594. Circulation. 1995. PMID: 7586362
-
Cytomegalovirus infection enhances mRNA expression of platelet-derived growth factor-BB and transforming growth factor-beta 1 in rat aortic allografts. Possible mechanism for cytomegalovirus-enhanced graft arteriosclerosis.Arterioscler Thromb. 1994 Dec;14(12):2043-52. doi: 10.1161/01.atv.14.12.2043. Arterioscler Thromb. 1994. PMID: 7981194
-
Triple drug immunosuppression significantly reduces immune activation and allograft arteriosclerosis in cytomegalovirus-infected rat aortic allografts and induces early latency of viral infection.Am J Pathol. 1994 Jun;144(6):1334-47. Am J Pathol. 1994. PMID: 8203471 Free PMC article.
-
Cytomegalovirus infection and cardiac allograft vasculopathy.Transpl Infect Dis. 1999 Jun;1(2):115-26. doi: 10.1034/j.1399-3062.1999.010205.x. Transpl Infect Dis. 1999. PMID: 11428979 Review.
-
Morphology and immunohistochemistry of rat aortic grafts.Folia Microbiol (Praha). 1999;44(3):339-53. doi: 10.1007/BF02818558. Folia Microbiol (Praha). 1999. PMID: 10664891 Review.
Cited by
-
Infection of vascular endothelial cells with human cytomegalovirus under fluid shear stress reveals preferential entry and spread of virus in flow conditions simulating atheroprone regions of the artery.J Virol. 2012 Dec;86(24):13745-55. doi: 10.1128/JVI.02244-12. Epub 2012 Oct 10. J Virol. 2012. PMID: 23055562 Free PMC article.
-
Cytomegalovirus and HIV: A Dangerous Pas de Deux.J Infect Dis. 2016 Oct 1;214 Suppl 2(Suppl 2):S67-74. doi: 10.1093/infdis/jiw217. J Infect Dis. 2016. PMID: 27625433 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

