Phosphorylation of Mycobacterium tuberculosis Ser/Thr phosphatase by PknA and PknB
- PMID: 21423706
- PMCID: PMC3052367
- DOI: 10.1371/journal.pone.0017871
Phosphorylation of Mycobacterium tuberculosis Ser/Thr phosphatase by PknA and PknB
Abstract
Background: The integrated functions of 11 Ser/Thr protein kinases (STPKs) and one phosphatase manipulate the phosphorylation levels of critical proteins in Mycobacterium tuberculosis. In this study, we show that the lone Ser/Thr phosphatase (PstP) is regulated through phosphorylation by STPKs.
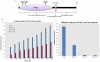
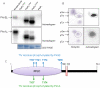
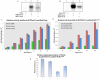
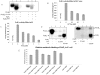
Principal findings: PstP is phosphorylated by PknA and PknB and phosphorylation is influenced by the presence of Zn(2+)-ions and inorganic phosphate (Pi). PstP is differentially phosphorylated on the cytosolic domain with Thr(137), Thr(141), Thr(174) and Thr(290) being the target residues of PknB while Thr(137) and Thr(174) are phosphorylated by PknA. The Mn(2+)-ion binding residues Asp(38) and Asp(229) are critical for the optimal activity of PstP and substitution of these residues affects its phosphorylation status. Native PstP and its phosphatase deficient mutant PstP(c) (D38G) are phosphorylated by PknA and PknB in E. coli and addition of Zn(2+)/Pi in the culture conditions affect the phosphorylation level of PstP. Interestingly, the phosphorylated phosphatase is more active than its unphosphorylated equivalent.
Conclusions and significance: This study establishes the novel mechanisms for regulation of mycobacterial Ser/Thr phosphatase. The results indicate that STPKs and PstP may regulate the signaling through mutually dependent mechanisms. Consequently, PstP phosphorylation may play a critical role in regulating its own activity. Since, the equilibrium between phosphorylated and non-phosphorylated states of mycobacterial proteins is still unexplained, understanding the regulation of PstP may help in deciphering the signal transduction pathways mediated by STPKs and the reversibility of the phenomena.
Conflict of interest statement
Figures

Similar articles
-
PknB kinase activity is regulated by phosphorylation in two Thr residues and dephosphorylation by PstP, the cognate phospho-Ser/Thr phosphatase, in Mycobacterium tuberculosis.Mol Microbiol. 2003 Sep;49(6):1493-508. doi: 10.1046/j.1365-2958.2003.03657.x. Mol Microbiol. 2003. PMID: 12950916
-
Phosphoprotein phosphatase of Mycobacterium tuberculosis dephosphorylates serine-threonine kinases PknA and PknB.Biochem Biophys Res Commun. 2003 Nov 7;311(1):112-20. doi: 10.1016/j.bbrc.2003.09.173. Biochem Biophys Res Commun. 2003. PMID: 14575702
-
Evidence that phosphorylation of threonine in the GT motif triggers activation of PknA, a eukaryotic-type serine/threonine kinase from Mycobacterium tuberculosis.FEBS J. 2015 Apr;282(8):1419-31. doi: 10.1111/febs.13230. Epub 2015 Mar 2. FEBS J. 2015. PMID: 25665034
-
Targeting the messengers: Serine/threonine protein kinases as potential targets for antimycobacterial drug development.IUBMB Life. 2018 Sep;70(9):889-904. doi: 10.1002/iub.1871. Epub 2018 Jun 22. IUBMB Life. 2018. PMID: 29934969 Review.
-
Structure/function studies of Ser/Thr and Tyr protein phosphorylation in Mycobacterium tuberculosis.J Mol Microbiol Biotechnol. 2005;9(3-4):167-81. doi: 10.1159/000089645. J Mol Microbiol Biotechnol. 2005. PMID: 16415590 Review.
Cited by
-
Ser/Thr phosphorylation as a regulatory mechanism in bacteria.Curr Opin Microbiol. 2015 Apr;24:47-52. doi: 10.1016/j.mib.2015.01.005. Epub 2015 Jan 24. Curr Opin Microbiol. 2015. PMID: 25625314 Free PMC article. Review.
-
Mycobacterium tuberculosis Serine/Threonine Protein Kinases.Microbiol Spectr. 2014 Oct;2(5):10.1128/microbiolspec.MGM2-0006-2013. doi: 10.1128/microbiolspec.MGM2-0006-2013. Microbiol Spectr. 2014. PMID: 25429354 Free PMC article. Review.
-
Unveiling the novel dual specificity protein kinases in Bacillus anthracis: identification of the first prokaryotic dual specificity tyrosine phosphorylation-regulated kinase (DYRK)-like kinase.J Biol Chem. 2012 Aug 3;287(32):26749-63. doi: 10.1074/jbc.M112.351304. Epub 2012 Jun 18. J Biol Chem. 2012. PMID: 22711536 Free PMC article.
-
Interaction of Mycobacterium tuberculosis elongation factor Tu with GTP is regulated by phosphorylation.J Bacteriol. 2011 Oct;193(19):5347-58. doi: 10.1128/JB.05469-11. Epub 2011 Jul 29. J Bacteriol. 2011. PMID: 21803988 Free PMC article.
-
Mycobacterial phosphatase PstP regulates global serine threonine phosphorylation and cell division.Sci Rep. 2019 Jun 6;9(1):8337. doi: 10.1038/s41598-019-44841-9. Sci Rep. 2019. PMID: 31171861 Free PMC article.
References
-
- Galyov EE, Hakansson S, Forsberg A, Wolf-Watz H. A secreted protein kinase of Yersinia pseudotuberculosis is an indispensable virulence determinant. Nature. 1993;361:730–732. - PubMed
-
- Bach H, Wong D, Av-Gay Y. Mycobacterium tuberculosis PtkA is a novel protein tyrosine kinase whose substrate is PtpA. Biochem J. 2009;420:155–160. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

