Ascaroside expression in Caenorhabditis elegans is strongly dependent on diet and developmental stage
- PMID: 21423575
- PMCID: PMC3058051
- DOI: 10.1371/journal.pone.0017804
Ascaroside expression in Caenorhabditis elegans is strongly dependent on diet and developmental stage
Abstract
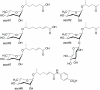
Background: The ascarosides form a family of small molecules that have been isolated from cultures of the nematode Caenorhabditis elegans. They are often referred to as "dauer pheromones" because most of them induce formation of long-lived and highly stress resistant dauer larvae. More recent studies have shown that ascarosides serve additional functions as social signals and mating pheromones. Thus, ascarosides have multiple functions. Until now, it has been generally assumed that ascarosides are constitutively expressed during nematode development.
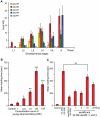
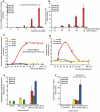
Methodology/principal findings: Cultures of C. elegans were developmentally synchronized on controlled diets. Ascarosides released into the media, as well as stored internally, were quantified by LC/MS. We found that ascaroside biosynthesis and release were strongly dependent on developmental stage and diet. The male attracting pheromone was verified to be a blend of at least four ascarosides, and peak production of the two most potent mating pheromone components, ascr#3 and asc#8 immediately preceded or coincided with the temporal window for mating. The concentration of ascr#2 increased under starvation conditions and peaked during dauer formation, strongly supporting ascr#2 as the main population density signal (dauer pheromone). After dauer formation, ascaroside production largely ceased and dauer larvae did not release any ascarosides. These findings show that both total ascaroside production and the relative proportions of individual ascarosides strongly correlate with these compounds' stage-specific biological functions.
Conclusions/significance: Ascaroside expression changes with development and environmental conditions. This is consistent with multiple functions of these signaling molecules. Knowledge of such differential regulation will make it possible to associate ascaroside production to gene expression profiles (transcript, protein or enzyme activity) and help to determine genetic pathways that control ascaroside biosynthesis. In conjunction with findings from previous studies, our results show that the pheromone system of C. elegans mimics that of insects in many ways, suggesting that pheromone signaling in C. elegans may exhibit functional homology also at the sensory level. In addition, our results provide a strong foundation for future behavioral modeling studies.
Conflict of interest statement
Figures
Similar articles
-
Ascaroside activity in Caenorhabditis elegans is highly dependent on chemical structure.Bioorg Med Chem. 2013 Sep 15;21(18):5754-69. doi: 10.1016/j.bmc.2013.07.018. Epub 2013 Jul 18. Bioorg Med Chem. 2013. PMID: 23920482 Free PMC article.
-
Formation and function of dauer ascarosides in the nematodes Caenorhabditis briggsae and Caenorhabditis elegans.G3 (Bethesda). 2022 Mar 4;12(3):jkac014. doi: 10.1093/g3journal/jkac014. G3 (Bethesda). 2022. PMID: 35094091 Free PMC article.
-
A blend of small molecules regulates both mating and development in Caenorhabditis elegans.Nature. 2008 Aug 28;454(7208):1115-8. doi: 10.1038/nature07168. Epub 2008 Jul 23. Nature. 2008. PMID: 18650807 Free PMC article.
-
Ascaroside Pheromones: Chemical Biology and Pleiotropic Neuronal Functions.Int J Mol Sci. 2019 Aug 9;20(16):3898. doi: 10.3390/ijms20163898. Int J Mol Sci. 2019. PMID: 31405082 Free PMC article. Review.
-
Ascaroside signaling in C. elegans.WormBook. 2013 Jan 18:1-22. doi: 10.1895/wormbook.1.155.1. WormBook. 2013. PMID: 23355522 Free PMC article. Review.
Cited by
-
A Single-Neuron Chemosensory Switch Determines the Valence of a Sexually Dimorphic Sensory Behavior.Curr Biol. 2018 Mar 19;28(6):902-914.e5. doi: 10.1016/j.cub.2018.02.029. Epub 2018 Mar 8. Curr Biol. 2018. PMID: 29526590 Free PMC article.
-
Ascaroside signaling is widely conserved among nematodes.Curr Biol. 2012 May 8;22(9):772-80. doi: 10.1016/j.cub.2012.03.024. Epub 2012 Apr 12. Curr Biol. 2012. PMID: 22503501 Free PMC article.
-
Targeted metabolomics reveals a male pheromone and sex-specific ascaroside biosynthesis in Caenorhabditis elegans.ACS Chem Biol. 2012 Aug 17;7(8):1321-5. doi: 10.1021/cb300169c. Epub 2012 Jun 12. ACS Chem Biol. 2012. PMID: 22662967 Free PMC article.
-
Improved Synthesis for Modular Ascarosides Uncovers Biological Activity.Org Lett. 2017 Jun 2;19(11):2837-2840. doi: 10.1021/acs.orglett.7b01009. Epub 2017 May 17. Org Lett. 2017. PMID: 28513161 Free PMC article.
-
FMRFamide-like peptides expand the behavioral repertoire of a densely connected nervous system.Proc Natl Acad Sci U S A. 2017 Dec 12;114(50):E10726-E10735. doi: 10.1073/pnas.1710374114. Epub 2017 Nov 22. Proc Natl Acad Sci U S A. 2017. PMID: 29167374 Free PMC article.
References
-
- Barriere A, Felix MA. High local genetic diversity and low outcrossing rate in Caenorhabditis elegans natural populations. Curr Biol. 2005;15:1176–1184. - PubMed
-
- Golden JW, Riddle DL. The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food, and temperature. Dev Biol. 1984;102:368–378. - PubMed
-
- Golden JW, Riddle DL. A pheromone influences larval development in the nematode Caenorhabditis elegans. Science. 1982;218:578–580. - PubMed
-
- Jeong P-Y, Jung M, Yim Y-H, Kim H, Park M, et al. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature. 2005;433:541–545. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

