Interleukin-12 converts Foxp3+ regulatory T cells to interferon-γ-producing Foxp3+ T cells that inhibit colitis
- PMID: 21419767
- PMCID: PMC3109200
- DOI: 10.1053/j.gastro.2011.03.009
Interleukin-12 converts Foxp3+ regulatory T cells to interferon-γ-producing Foxp3+ T cells that inhibit colitis
Abstract
Background & aims: Regulatory T (Treg) cells are plastic, but the in vivo mechanisms by which they are converted into foxhead box p3 (Foxp3+) interferon (IFN)-γ+ T cells and whether these converted cells retain the ability to inhibit colitis are not clear.
Methods: Foxp3+ Treg cells were generated by culture of naïve CD4+ T cells from Foxp3GFP CBir1 T-cell receptor (TCR) transgenic (Tg) (CBir1-Tg) mice, which are specific for CBir1 flagellin (an immunodominant microbiota antigen), with transforming growth factor-β. Foxp3GFP+ CBir1-Tg Treg cells were isolated by fluorescence-activated cell sorting and transferred into TCRβxδ-/- mice. Colitis was induced by transfer of naïve CBir1-Tg CD4+ T cells into immunodeficient mice.
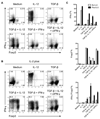
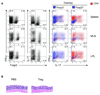
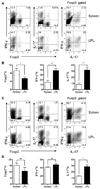
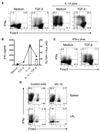
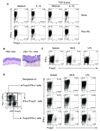
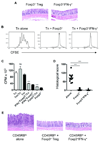
Results: Microbiota antigen-specific Foxp3+ Treg cells were converted, in the intestine, to IFN-γ+ T-helper (Th)1 cells, interleukin (IL)-17+ Th17 cells, and Foxp3+ T cells that coexpress IFN-γ and/or IL-17. Conversion of Treg cells into IFN-γ-producing Th1 cells and Foxp3+IFN-γ+ T cells required innate cell production of IL-12 in the intestine; blocking IL-12 with an antibody inhibited their conversion to Th1 and Foxp3+IFN-γ+ T cells in the intestines of mice that were recipients of Treg cells. Addition of IL-12, but not IL-23, promoted conversion of Treg cells into Th1 and Foxp3+IFN-γ+ T cells, in vitro. Foxp3+IFN-γ+ T cells had regulatory activity because they suppressed proliferation of naïve T cells, in vitro, and inhibited induction of colitis by microbiota antigen-specific T cells. IFN-γ+ Th1 cells were not converted into Treg cells; Foxp3+IFN-γ+ T cells differentiated into IFN-γ+ but not Foxp3+ T cells.
Conclusions: IL-12 promotes conversion of Treg cells into IFN-γ-expressing cells; Foxp3+IFN-γ+ T cells retain their regulatory functions and develop during the transition of Foxp3+ Treg cells into IFN-γ+ Th1 cells.
Copyright © 2011 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
No authors have conflicting financial interests.
Figures

Similar articles
-
TGF-β converts Th1 cells into Th17 cells through stimulation of Runx1 expression.Eur J Immunol. 2015 Apr;45(4):1010-8. doi: 10.1002/eji.201444726. Epub 2015 Feb 11. Eur J Immunol. 2015. PMID: 25605286 Free PMC article.
-
Th17 cells induce colitis and promote Th1 cell responses through IL-17 induction of innate IL-12 and IL-23 production.J Immunol. 2011 Jun 1;186(11):6313-8. doi: 10.4049/jimmunol.1001454. Epub 2011 Apr 29. J Immunol. 2011. PMID: 21531892 Free PMC article.
-
Antigen-presenting cell production of IL-10 inhibits T-helper 1 and 17 cell responses and suppresses colitis in mice.Gastroenterology. 2011 Aug;141(2):653-62, 662.e1-4. doi: 10.1053/j.gastro.2011.04.053. Epub 2011 Apr 30. Gastroenterology. 2011. PMID: 21679711 Free PMC article.
-
The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease.Clin Exp Immunol. 2007 Apr;148(1):32-46. doi: 10.1111/j.1365-2249.2007.03356.x. Clin Exp Immunol. 2007. PMID: 17328715 Free PMC article. Review.
-
Regulatory T cells and the induction of IL-17.Mucosal Immunol. 2008 Nov;1 Suppl 1:S43-6. doi: 10.1038/mi.2008.51. Mucosal Immunol. 2008. PMID: 19079228 Review.
Cited by
-
T-bet(+) Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor β2.Immunity. 2012 Sep 21;37(3):501-10. doi: 10.1016/j.immuni.2012.05.031. Epub 2012 Sep 6. Immunity. 2012. PMID: 22960221 Free PMC article.
-
Comparative study of subcutaneous, intramuscular, and oral administration of bovine pathogenic Escherichia coli bacterial ghost vaccine in mice.Front Immunol. 2022 Nov 14;13:1008131. doi: 10.3389/fimmu.2022.1008131. eCollection 2022. Front Immunol. 2022. PMID: 36451816 Free PMC article.
-
Induction of antitumor immunity ex vivo using dendritic cells transduced with fowl pox vector expressing MUC1, CEA, and a triad of costimulatory molecules (rF-PANVAC).J Immunother. 2012 Sep;35(7):555-69. doi: 10.1097/CJI.0b013e31826a73de. J Immunother. 2012. PMID: 22892452 Free PMC article.
-
The Alternative NF-κB Pathway in Regulatory T Cell Homeostasis and Suppressive Function.J Immunol. 2018 Apr 1;200(7):2362-2371. doi: 10.4049/jimmunol.1800042. Epub 2018 Feb 19. J Immunol. 2018. PMID: 29459403 Free PMC article.
-
Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion.Front Immunol. 2018 May 4;9:847. doi: 10.3389/fimmu.2018.00847. eCollection 2018. Front Immunol. 2018. PMID: 29780381 Free PMC article. Review.
References
-
- Strober W. The multifaceted influence of the mucosal microflora on mucosal dendritic cell responses. Immunity. 2009;31:377–388. - PubMed
-
- Barnes MJ, Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity. 2009;31:401–411. - PubMed
-
- Belkaid Y, Tarbell KV. Arming Treg cells at the inflammatory site. Immunity. 2009;30:322–323. - PubMed
-
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI083484/AI/NIAID NIH HHS/United States
- DK064400/DK/NIDDK NIH HHS/United States
- R01 DK079918-01A1/DK/NIDDK NIH HHS/United States
- R24 DK064400/DK/NIDDK NIH HHS/United States
- RR-20136/RR/NCRR NIH HHS/United States
- C06 RR020136/RR/NCRR NIH HHS/United States
- R01 DK079918/DK/NIDDK NIH HHS/United States
- DK079918/DK/NIDDK NIH HHS/United States
- P01 DK071176/DK/NIDDK NIH HHS/United States
- R21 AI083484-01/AI/NIAID NIH HHS/United States
- R21 AI083484/AI/NIAID NIH HHS/United States
- P01 DK071176-04/DK/NIDDK NIH HHS/United States
- DK071176/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

