Dopamine promotes formation and secretion of non-fibrillar alpha-synuclein oligomers
- PMID: 21415592
- PMCID: PMC3085740
- DOI: 10.3858/emm.2011.43.4.026
Dopamine promotes formation and secretion of non-fibrillar alpha-synuclein oligomers
Abstract
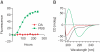
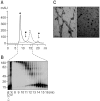
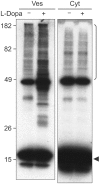
Parkinson's disease (PD) is characterized by selective and progressive degeneration of dopamine (DA)-producing neurons in the substantia nigra pars compacta (SNpc) and by abnormal aggregation of α-synuclein. Previous studies have suggested that DA can interact with α-synuclein, thus modulating the aggregation process of this protein; this interaction may account for the selective vulnerability of DA neurons in patients with PD. However, the relationship between DA and α-synuclein, and the role in progressive degeneration of DA neurons remains elusive. We have shown that in the presence of DA, recombinant human α-synuclein produces non-fibrillar, SDS-resistant oligomers, while β-sheet-rich fibril formation is inhibited. Pharmacologic elevation of the cytoplasmic DA level increased the formation of SDS-resistant oligomers in DA-producing neuronal cells. DA promoted α-synuclein oligomerization in intracellular vesicles, but not in the cytosol. Furthermore, elevation of DA levels increased secretion of α-synuclein oligomers to the extracellular space, but the secretion of monomers was not changed. DA-induced secretion of α-synuclein oligomers may contribute to the progressive loss of the dopaminergic neuronal population and the pronounced neuroinflammation observed in the SNpc in patients with PD.
Figures
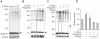

Similar articles
-
Aldehyde dehydrogenase 1 defines and protects a nigrostriatal dopaminergic neuron subpopulation.J Clin Invest. 2014 Jul;124(7):3032-46. doi: 10.1172/JCI72176. Epub 2014 May 27. J Clin Invest. 2014. PMID: 24865427 Free PMC article.
-
Dopamine facilitates alpha-synuclein oligomerization in human neuroblastoma SH-SY5Y cells.Biochem Biophys Res Commun. 2010 Jan 1;391(1):129-34. doi: 10.1016/j.bbrc.2009.11.015. Epub 2009 Nov 11. Biochem Biophys Res Commun. 2010. PMID: 19900407
-
The role of alpha-synuclein in the development of the dopaminergic neurons in the substantia nigra and ventral tegmental area.Dokl Biol Sci. 2016;466:5-7. doi: 10.1134/S0012496616010117. Epub 2016 Mar 30. Dokl Biol Sci. 2016. PMID: 27021360
-
Interaction of alpha-synuclein and dopamine metabolites in the pathogenesis of Parkinson's disease: a case for the selective vulnerability of the substantia nigra.Acta Neuropathol. 2006 Aug;112(2):115-26. doi: 10.1007/s00401-006-0096-2. Epub 2006 Jun 22. Acta Neuropathol. 2006. PMID: 16791599 Review.
-
Modulation of alpha-synuclein aggregation by dopamine: a review.Neurochem Res. 2009 Oct;34(10):1838-46. doi: 10.1007/s11064-009-9986-8. Epub 2009 May 15. Neurochem Res. 2009. PMID: 19444607 Review.
Cited by
-
Caffeine reduces deficits in mechanosensation and locomotion induced by L-DOPA and protects dopaminergic neurons in a transgenic Caenorhabditis elegans model of Parkinson's disease.Pharm Biol. 2020 Dec;58(1):721-731. doi: 10.1080/13880209.2020.1791192. Pharm Biol. 2020. PMID: 32715838 Free PMC article.
-
Parkinson's Disease: Biomarkers, Treatment, and Risk Factors.Front Neurosci. 2018 Aug 30;12:612. doi: 10.3389/fnins.2018.00612. eCollection 2018. Front Neurosci. 2018. PMID: 30214392 Free PMC article. Review.
-
β1-integrin-dependent migration of microglia in response to neuron-released α-synuclein.Exp Mol Med. 2014 Apr 18;46(4):e91. doi: 10.1038/emm.2014.6. Exp Mol Med. 2014. PMID: 24743837 Free PMC article.
-
What's to like about the prion-like hypothesis for the spreading of aggregated α-synuclein in Parkinson disease?Prion. 2013 Jan-Feb;7(1):92-7. doi: 10.4161/pri.23806. Epub 2013 Jan 1. Prion. 2013. PMID: 23360753 Free PMC article. Review.
-
Alpha-Synuclein Strain Variability in Body-First and Brain-First Synucleinopathies.Front Aging Neurosci. 2022 May 26;14:907293. doi: 10.3389/fnagi.2022.907293. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35693346 Free PMC article.
References
-
- Bisaglia M, Mammi S, Bubacco L. Kinetic and structural analysis of the early oxidation products of dopamine: analysis of the interactions with {alpha}- synuclein. J Biol Chem. 2007;282:15597–15605. - PubMed
-
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. - PubMed
-
- Burke WJ, Kumar VB, Pandey N, Panneton WM, Gan Q, Franko MW, O'Dell M, Li SW, Pan Y, Chung HD, Galvin JE. Aggregation of alpha-synuclein by DOPAL, the monoamine oxidase metabolite of dopamine. Acta Neuropathol. 2008;115:193–203. - PubMed
-
- Cappai R, Leck SL, Tew DJ, Williamson NA, Smith DP, Galatis D, Sharples RA, Curtain CC, Ali FE, Cherny RA, Culvenor JG, Bottomley SP, Masters CL, Barnham KJ, Hill AF. Dopamine promotes alpha-synuclein aggregation into SDS-resistant soluble oligomers via a distinct folding pathway. FASEB J. 2005;19:1377–1379. - PubMed
-
- Conway KA, Rochet JC, Bieganski RM, Lansbury PT., Jr Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science. 2001;294:1346–1349. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
