Imaging light responses of targeted neuron populations in the rodent retina
- PMID: 21414907
- PMCID: PMC3521507
- DOI: 10.1523/JNEUROSCI.6064-10.2011
Imaging light responses of targeted neuron populations in the rodent retina
Abstract
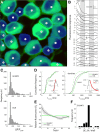
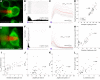
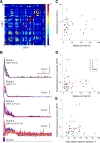
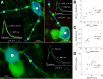
Decoding the wiring diagram of the retina requires simultaneous observation of activity in identified neuron populations. Available recording methods are limited in their scope: electrodes can access only a small fraction of neurons at once, whereas synthetic fluorescent indicator dyes label tissue indiscriminately. Here, we describe a method for studying retinal circuitry at cellular and subcellular levels combining two-photon microscopy and a genetically encoded calcium indicator. Using specific viral and promoter constructs to drive expression of GCaMP3, we labeled all five major neuron classes in the adult mouse retina. Stimulus-evoked GCaMP3 responses as imaged by two-photon microscopy permitted functional cell type annotation. Fluorescence responses were similar to those measured with the small molecule dye OGB-1. Fluorescence intensity correlated linearly with spike rates >10 spikes/s, and a significant change in fluorescence always reflected a significant change in spike firing rate. GCaMP3 expression had no apparent effect on neuronal function. Imaging at subcellular resolution showed compartment-specific calcium dynamics in multiple identified cell types.
Figures

Similar articles
-
Fast two-photon imaging of subcellular voltage dynamics in neuronal tissue with genetically encoded indicators.Elife. 2017 Jul 27;6:e25690. doi: 10.7554/eLife.25690. Elife. 2017. PMID: 28749338 Free PMC article.
-
Imaging Voltage in Genetically Defined Neuronal Subpopulations with a Cre Recombinase-Targeted Hybrid Voltage Sensor.J Neurosci. 2017 Sep 20;37(38):9305-9319. doi: 10.1523/JNEUROSCI.1363-17.2017. Epub 2017 Aug 23. J Neurosci. 2017. PMID: 28842412 Free PMC article.
-
Light responses and morphology of bNOS-immunoreactive neurons in the mouse retina.J Comp Neurol. 2010 Jul 1;518(13):2456-74. doi: 10.1002/cne.22347. J Comp Neurol. 2010. PMID: 20503422 Free PMC article.
-
Voltage imaging with ANNINE dyes and two-photon microscopy of Purkinje dendrites in awake mice.Neurosci Res. 2020 Mar;152:15-24. doi: 10.1016/j.neures.2019.11.007. Epub 2019 Nov 20. Neurosci Res. 2020. PMID: 31758973 Review.
-
In Vivo Observations of Rapid Scattered Light Changes Associated with Neurophysiological Activity.In: Frostig RD, editor. In Vivo Optical Imaging of Brain Function. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 5. In: Frostig RD, editor. In Vivo Optical Imaging of Brain Function. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 5. PMID: 26844322 Free Books & Documents. Review.
Cited by
-
The dynamic receptive fields of retinal ganglion cells.Prog Retin Eye Res. 2018 Nov;67:102-117. doi: 10.1016/j.preteyeres.2018.06.003. Epub 2018 Jun 23. Prog Retin Eye Res. 2018. PMID: 29944919 Free PMC article. Review.
-
Glial Cell Calcium Signaling Mediates Capillary Regulation of Blood Flow in the Retina.J Neurosci. 2016 Sep 7;36(36):9435-45. doi: 10.1523/JNEUROSCI.1782-16.2016. J Neurosci. 2016. PMID: 27605617 Free PMC article.
-
Stimulation strategies for selective activation of retinal ganglion cell soma and threshold reduction.J Neural Eng. 2019 Apr;16(2):026017. doi: 10.1088/1741-2552/aaf92b. Epub 2018 Dec 18. J Neural Eng. 2019. PMID: 30560810 Free PMC article.
-
Imaging single cells in the living retina.Vision Res. 2011 Jul 1;51(13):1379-96. doi: 10.1016/j.visres.2011.05.002. Epub 2011 May 10. Vision Res. 2011. PMID: 21596053 Free PMC article. Review.
-
An optimized fluorescent probe for visualizing glutamate neurotransmission.Nat Methods. 2013 Feb;10(2):162-70. doi: 10.1038/nmeth.2333. Epub 2013 Jan 13. Nat Methods. 2013. PMID: 23314171 Free PMC article.
References
-
- Barlow HB, Levick WR, Yoon M. Responses to single quanta of light in retinal ganglion cells of the cat. Vision Res. 1971;1971(Suppl 3):87–101. - PubMed
-
- Casagrande VA, Xu X, editors. Parallel visual pathways: a comparative perspective. Cambridge, MA: MIT; 2004.
-
- Chichilnisky EJ. A simple white noise analysis of neuronal light responses. Network. 2001;12:199–213. - PubMed
-
- Demb JB. Cellular mechanisms for direction selectivity in the retina. Neuron. 2007;55:179–186. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
