Aging and a peripheral immune challenge interact to reduce mature brain-derived neurotrophic factor and activation of TrkB, PLCgamma1, and ERK in hippocampal synaptoneurosomes
- PMID: 21411668
- PMCID: PMC3086395
- DOI: 10.1523/JNEUROSCI.5818-10.2011
Aging and a peripheral immune challenge interact to reduce mature brain-derived neurotrophic factor and activation of TrkB, PLCgamma1, and ERK in hippocampal synaptoneurosomes
Abstract
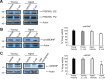
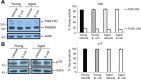
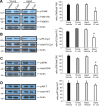
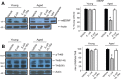
For reasons that are not well understood, aging significantly increases brain vulnerability to challenging life events. High-functioning older individuals often experience significant cognitive decline after an inflammatory event such as surgery, infection, or injury. We have modeled this phenomenon in rodents and have previously reported that a peripheral immune challenge (intraperitoneal injection of live Escherichia coli) selectively disrupts consolidation of hippocampus-dependent memory in aged (24-month-old), but not young (3-month-old), F344xBN rats. More recently, we have demonstrated that this infection-evoked memory deficit is mirrored by a selective deficit in long-lasting synaptic plasticity in the hippocampus. Interestingly, these deficits occur in forms of long-term memory and synaptic plasticity known to be strongly dependent on brain-derived neurotrophic factor (BDNF). Here, we begin to test the hypothesis that the combination of aging and an infection might disrupt production or processing of BDNF protein in the hippocampus, decreasing the availability of BDNF for plasticity-related processes at synaptic sites. We find that mature BDNF is markedly reduced in Western blots of hippocampal synaptoneurosomes prepared from aged animals following infection. This reduction is blocked by intra-cisterna magna administration of the anti-inflammatory cytokine IL-1Ra (interleukin 1-specific receptor antagonist). Levels of the pan-neurotrophin receptor p75(NTR) and the BDNF receptor TrkB (tropomyosin receptor kinase B) are not significantly altered in these synaptoneurosomes, but phosphorylation of TrkB and downstream activation of PLCγ1 (phospholipase Cγ1) and ERK (extracellular response kinase) are attenuated-observations consistent with reduced availability of mature BDNF to activate TrkB signaling. These data suggest that inflammation-evoked reductions in BDNF at synapses might contribute to inflammation-evoked disruptions in long-term memory and synaptic plasticity in aging.
Figures
Similar articles
-
Aging and an Immune Challenge Interact to Produce Prolonged, but Not Permanent, Reductions in Hippocampal L-LTP and mBDNF in a Rodent Model with Features of Delirium.eNeuro. 2018 Jun 4;5(3):ENEURO.0009-18.2018. doi: 10.1523/ENEURO.0009-18.2018. eCollection 2018 May-Jun. eNeuro. 2018. PMID: 29911174 Free PMC article.
-
Investigating the effect of crocin on memory deficits induced by total sleep deprivation (TSD) with respect to the BDNF, TrkB and ERK levels in the hippocampus of male Wistar rats.J Psychopharmacol. 2021 Jun;35(6):744-754. doi: 10.1177/02698811211000762. Epub 2021 Apr 25. J Psychopharmacol. 2021. PMID: 33899577
-
Activation of adenosine A2A receptors induces TrkB translocation and increases BDNF-mediated phospho-TrkB localization in lipid rafts: implications for neuromodulation.J Neurosci. 2010 Jun 23;30(25):8468-80. doi: 10.1523/JNEUROSCI.5695-09.2010. J Neurosci. 2010. Retraction in: J Neurosci. 2013 Jan 16;33(3):1292. doi: 10.1523/JNEUROSCI.5704-12.2013 PMID: 20573894 Free PMC article. Retracted.
-
BDNF-induced local protein synthesis and synaptic plasticity.Neuropharmacology. 2014 Jan;76 Pt C:639-56. doi: 10.1016/j.neuropharm.2013.04.005. Epub 2013 Apr 16. Neuropharmacology. 2014. PMID: 23602987 Review.
-
BDNF and Hippocampal Synaptic Plasticity.Vitam Horm. 2017;104:153-195. doi: 10.1016/bs.vh.2016.10.004. Epub 2016 Nov 29. Vitam Horm. 2017. PMID: 28215294 Review.
Cited by
-
Memory-related hippocampal brain-derived neurotrophic factor activation pathways from repetitive transcranial magnetic stimulation in the 3xTg-AD mouse line.Exp Gerontol. 2023 Nov;183:112323. doi: 10.1016/j.exger.2023.112323. Epub 2023 Nov 1. Exp Gerontol. 2023. PMID: 39351497 Free PMC article.
-
The Role of Neurotrophin Signaling in Age-Related Cognitive Decline and Cognitive Diseases.Int J Mol Sci. 2022 Jul 13;23(14):7726. doi: 10.3390/ijms23147726. Int J Mol Sci. 2022. PMID: 35887075 Free PMC article. Review.
-
Enhanced neuroinflammation mediated by DNA methylation of the glucocorticoid receptor triggers cognitive dysfunction after sevoflurane anesthesia in adult rats subjected to maternal separation during the neonatal period.J Neuroinflammation. 2017 Jan 7;14(1):6. doi: 10.1186/s12974-016-0782-5. J Neuroinflammation. 2017. PMID: 28086911 Free PMC article.
-
Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications.Neuroscience. 2013 Aug 29;246:199-229. doi: 10.1016/j.neuroscience.2013.04.060. Epub 2013 May 3. Neuroscience. 2013. PMID: 23644052 Free PMC article. Review.
-
Male-specific effects of lipopolysaccharide on glucocorticoid receptor nuclear translocation in the prefrontal cortex of depressive rats.Psychopharmacology (Berl). 2016 Sep;233(18):3315-30. doi: 10.1007/s00213-016-4374-y. Epub 2016 Jul 7. Psychopharmacology (Berl). 2016. PMID: 27387895
References
-
- Barker PA. Whither proBDNF? Nat Neurosci. 2009;12:105–106. - PubMed
-
- Barrientos RM, Sprunger DB, Campeau S, Higgins EA, Watkins LR, Rudy JW, Maier SF. Brain-derived neurotrophic factor mRNA downregulation produced by social isolation is blocked by intrahippocampal interleukin-1 receptor antagonist. Neuroscience. 2003;121:847–853. - PubMed
-
- Barrientos RM, Sprunger DB, Campeau S, Watkins LR, Rudy JW, Maier SF. BDNF mRNA expression in rat hippocampus following contextual learning is blocked by intrahippocampal IL-1beta administration. J Neuroimmunol. 2004;155:119–126. - PubMed
-
- Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27:723–732. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
