Rapid and contrasting effects of rosiglitazone on transient receptor potential TRPM3 and TRPC5 channels
- PMID: 21406603
- PMCID: PMC3102547
- DOI: 10.1124/mol.110.069922
Rapid and contrasting effects of rosiglitazone on transient receptor potential TRPM3 and TRPC5 channels
Abstract
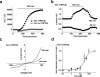
The aim of this study was to generate new insight into chemical regulation of transient receptor potential (TRP) channels with relevance to glucose homeostasis and the metabolic syndrome. Human TRP melastatin 2 (TRPM2), TRPM3, and TRP canonical 5 (TRPC5) were conditionally overexpressed in human embryonic kidney 293 cells and studied by using calcium-measurement and patch-clamp techniques. Rosiglitazone and other peroxisome proliferator-activated receptor-γ (PPAR-γ) agonists were investigated. TRPM2 was unaffected by rosiglitazone at concentrations up to 10 μM but was inhibited completely at higher concentrations (IC(50), ∼22.5 μM). TRPM3 was more potently inhibited, with effects occurring in a biphasic concentration-dependent manner such that there was approximately 20% inhibition at low concentrations (0.1-1 μM) and full inhibition at higher concentrations (IC(50), 5-10 μM). PPAR-γ antagonism by 2-chloro-5-nitrobenzanilide (GW9662) did not prevent inhibition of TRPM3 by rosiglitazone. TRPC5 was strongly stimulated by rosiglitazone at concentrations of ≥10 μM (EC(50), ∼30 μM). Effects on TRPM3 and TRPC5 occurred rapidly and reversibly. Troglitazone and pioglitazone inhibited TRPM3 (IC(50), 12 μM) but lacked effect on TRPC5, suggesting no relevance of PPAR-γ or the thiazolidinedione moiety to rosiglitazone stimulation of TRPC5. A rosiglitazone-related but nonthiazolidinedione PPAR-γ agonist, N-(2-benzoylphenyl)-O-[2-(methyl-2-pyridinylamino)ethyl]-l-tyrosine (GW1929), was a weak stimulator of TRPM3 and TRPC5. The natural PPAR-γ agonist 15-deoxy prostaglandin J(2), had no effect on TRPM3 or TRPC5. The data suggest that rosiglitazone contains chemical moieties that rapidly, strongly, and differentially modulate TRP channels independently of PPAR-γ, potentially contributing to biological consequences of the agent and providing the basis for novel TRP channel pharmacology.
Figures
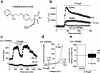
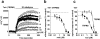

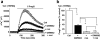


Similar articles
-
TRPM3 in temperature sensing and beyond.Temperature (Austin). 2015 Feb 25;2(2):201-13. doi: 10.4161/23328940.2014.988524. eCollection 2015 Apr-Jun. Temperature (Austin). 2015. PMID: 27227024 Free PMC article. Review.
-
Inhibition of endothelial cell Ca²⁺ entry and transient receptor potential channels by Sigma-1 receptor ligands.Br J Pharmacol. 2013 Mar;168(6):1445-55. doi: 10.1111/bph.12041. Br J Pharmacol. 2013. PMID: 23121507 Free PMC article.
-
Modulation of TRPC5 cation channels by halothane, chloroform and propofol.Br J Pharmacol. 2008 Apr;153(7):1505-12. doi: 10.1038/sj.bjp.0707689. Epub 2008 Jan 21. Br J Pharmacol. 2008. PMID: 18204473 Free PMC article.
-
Stimulation of TRPC5 cationic channels by low micromolar concentrations of lead ions (Pb2+).Biochem Biophys Res Commun. 2010 Feb 26;393(1):50-4. doi: 10.1016/j.bbrc.2010.01.074. Epub 2010 Jan 25. Biochem Biophys Res Commun. 2010. PMID: 20100462 Free PMC article.
-
Transient receptor potential TRPM3 channels: Pharmacology, signaling, and biological functions.Pharmacol Res. 2017 Oct;124:92-99. doi: 10.1016/j.phrs.2017.07.014. Epub 2017 Jul 16. Pharmacol Res. 2017. PMID: 28720517 Review.
Cited by
-
TRPM3 in temperature sensing and beyond.Temperature (Austin). 2015 Feb 25;2(2):201-13. doi: 10.4161/23328940.2014.988524. eCollection 2015 Apr-Jun. Temperature (Austin). 2015. PMID: 27227024 Free PMC article. Review.
-
A Review on the Role of TRP Channels and Their Potential as Drug Targets_An Insight Into the TRP Channel Drug Discovery Methodologies.Front Pharmacol. 2022 May 24;13:914499. doi: 10.3389/fphar.2022.914499. eCollection 2022. Front Pharmacol. 2022. PMID: 35685622 Free PMC article. Review.
-
Canonical transient receptor potential channels and their modulators: biology, pharmacology and therapeutic potentials.Arch Pharm Res. 2021 Apr;44(4):354-377. doi: 10.1007/s12272-021-01319-5. Epub 2021 Mar 24. Arch Pharm Res. 2021. PMID: 33763843 Free PMC article. Review.
-
TRPM3, TRPM4, and TRPM5 as thermo-sensitive channels.J Physiol Sci. 2024 Sep 18;74(1):43. doi: 10.1186/s12576-024-00937-0. J Physiol Sci. 2024. PMID: 39294615 Free PMC article. Review.
-
Diclofenac, a nonsteroidal anti-inflammatory drug, is an antagonist of human TRPM3 isoforms.Pharmacol Res Perspect. 2016 Apr 7;4(3):e00232. doi: 10.1002/prp2.232. eCollection 2016 Jun. Pharmacol Res Perspect. 2016. PMID: 27433342 Free PMC article.
References
-
- Ahn HS, Kim SE, Jang HJ, Kim MJ, Rhie DJ, Yoon SH, Jo YH, Kim MS, Sung KW, Kim SY, et al. (2007) Open channel block of Kv1.3 by rosiglitazone and troglitazone: Kv1.3 as the pharmacological target for rosiglitazone. Naunyn Schmiedebergs Arch Pharmacol 374:305–309 - PubMed
-
- Aramwit P, Supasyndh O, Sriboonruang T. (2008) Pharmacokinetics of single-dose rosiglitazone in chronic ambulatory peritoneal dialysis patients. J Clin Pharm Ther 33:685–690 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
