PvRON2, a new Plasmodium vivax rhoptry neck antigen
- PMID: 21401956
- PMCID: PMC3068128
- DOI: 10.1186/1475-2875-10-60
PvRON2, a new Plasmodium vivax rhoptry neck antigen
Abstract
Background: Rhoptries are specialized organelles from parasites belonging to the phylum Apicomplexa; they secrete their protein content during invasion of host target cells and are sorted into discrete subcompartments within rhoptry neck or bulb. This distribution is associated with these proteins' role in tight junction (TJ) and parasitophorous vacuole (PV) formation, respectively.
Methods: Plasmodium falciparum RON2 amino acid sequence was used as bait for screening the codifying gene for the homologous protein in the Plasmodium vivax genome. Gene synteny, as well as identity and similarity values, were determined for ron2 and its flanking genes among P. falciparum, P. vivax and other malarial parasite genomes available at PlasmoDB and Sanger Institute databases. Pvron2 gene transcription was determined by RT-PCR of cDNA obtained from the P. vivax VCG-1 strain. Protein expression and localization were assessed by Western blot and immunofluorescence using polyclonal anti-PvRON2 antibodies. Co-localization was confirmed using antibodies directed towards specific microneme and rhoptry neck proteins.
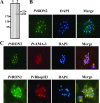
Results and discussion: The first P. vivax rhoptry neck protein (named here PvRON2) has been identified in this study. PvRON2 is a 2,204 residue-long protein encoded by a single 6,615 bp exon containing a hydrophobic signal sequence towards the amino-terminus, a transmembrane domain towards the carboxy-terminus and two coiled coil α-helical motifs; these are characteristic features of several previously described vaccine candidates against malaria. This protein also contains two tandem repeats within the interspecies variable sequence possibly involved in evading a host's immune system. PvRON2 is expressed in late schizonts and localized in rhoptry necks similar to what has been reported for PfRON2, which suggests its participation during target cell invasion.
Conclusions: The identification and partial characterization of the first P. vivax rhoptry neck protein are described in the present study. This protein is homologous to PfRON2 which has previously been shown to be associated with PfAMA-1, suggesting a similar role for PvRON2.
Figures

Similar articles
-
Identification, characterization and antigenicity of the Plasmodium vivax rhoptry neck protein 1 (PvRON1).Malar J. 2011 Oct 24;10:314. doi: 10.1186/1475-2875-10-314. Malar J. 2011. PMID: 22024312 Free PMC article.
-
Annotation and characterization of the Plasmodium vivax rhoptry neck protein 4 (PvRON4).Malar J. 2013 Oct 5;12:356. doi: 10.1186/1475-2875-12-356. Malar J. 2013. PMID: 24093777 Free PMC article.
-
The Plasmodium vivax rhoptry neck protein 5 is expressed in the apical pole of Plasmodium vivax VCG-1 strain schizonts and binds to human reticulocytes.Malar J. 2015 Mar 7;14:106. doi: 10.1186/s12936-015-0619-1. Malar J. 2015. PMID: 25888962 Free PMC article.
-
Structure of Rhoptry Neck Protein 2 is essential for the interaction in vitro with Apical Membrane Antigen 1 in Plasmodium vivax.Malar J. 2019 Jan 25;18(1):25. doi: 10.1186/s12936-019-2649-6. Malar J. 2019. PMID: 30683104 Free PMC article.
-
Apical membrane antigen 1 as an anti-malarial drug target.Curr Top Med Chem. 2011;11(16):2039-47. doi: 10.2174/156802611796575885. Curr Top Med Chem. 2011. PMID: 21619512 Review.
Cited by
-
Antigenicity and immunogenicity of PvRALP1, a novel Plasmodium vivax rhoptry neck protein.Malar J. 2015 Apr 29;14:186. doi: 10.1186/s12936-015-0698-z. Malar J. 2015. PMID: 25925592 Free PMC article.
-
Plasmodium vivax vaccine: What is the best way to go?Front Immunol. 2023 Jan 16;13:910236. doi: 10.3389/fimmu.2022.910236. eCollection 2022. Front Immunol. 2023. PMID: 36726991 Free PMC article. Review.
-
Molecular Cloning and Characterization of Babesia orientalis Rhoptry Neck 2 BoRON2 Protein.J Parasitol Res. 2017;2017:7259630. doi: 10.1155/2017/7259630. Epub 2017 Jul 9. J Parasitol Res. 2017. PMID: 28775897 Free PMC article.
-
The in Vitro Antigenicity of Plasmodium vivax Rhoptry Neck Protein 2 (PvRON2) B- and T-Epitopes Selected by HLA-DRB1 Binding Profile.Front Cell Infect Microbiol. 2018 May 15;8:156. doi: 10.3389/fcimb.2018.00156. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29868512 Free PMC article.
-
Identification of Plasmodium vivax proteins with potential role in invasion using sequence redundancy reduction and profile hidden Markov models.PLoS One. 2011;6(10):e25189. doi: 10.1371/journal.pone.0025189. Epub 2011 Oct 3. PLoS One. 2011. PMID: 21984903 Free PMC article.
References
-
- Krotoski WA, Garnham PC, Cogswell FB, Collins WE, Bray RS, Gwasz RW, Killick-Kendrick R, Wolf RH, Sinden R, Hollingdale M, Lowrie RC Jr, Koontz LC, Stanfill PS. Observations on early and late post-sporozoite tissue stages in primate malaria. IV. Pre-erythrocytic schizonts and/or hypnozoites of Chesson and North Korean strains of Plasmodium vivax in the chimpanzee. Am J Trop Med Hyg. 1986;35:263–274. - PubMed
-
- Bozdech Z, Mok S, Hu G, Imwong M, Jaidee A, Russell B, Ginsburg H, Nosten F, Day NP, White NJ, Carlton JM, Preiser PR. The transcriptome of Plasmodium vivax reveals divergence and diversity of transcriptional regulation in malaria parasites. Proc Natl Acad Sci USA. 2008;105:16290–16295. doi: 10.1073/pnas.0807404105. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

