The role of extracellular adenosine in chemical neurotransmission in the hippocampus and Basal Ganglia: pharmacological and clinical aspects
- PMID: 21401497
- PMCID: PMC3179034
- DOI: 10.2174/156802611795347564
The role of extracellular adenosine in chemical neurotransmission in the hippocampus and Basal Ganglia: pharmacological and clinical aspects
Abstract
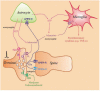
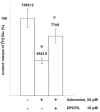
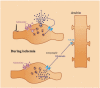
Now there is general agreement that the purine nucleoside adenosine is an important neuromodulator in the central nervous system, playing a crucial role in neuronal excitability and synaptic/non-synaptic transmission in the hippocampus and basal ganglia. Adenosine is derived from the breakdown of extra- or intracellular ATP and is released upon a variety of physiological and pathological stimuli from neuronal and non-neuronal sources, i.e. from glial cells and exerts effects diffusing far away from release sites. The resultant elevation of adenosine levels in the extracellular space reaches micromolar level, and leads to the activation A(1), A(2A), A(2B) and A(3) receptors, localized to pre- and postsynaptic as well as extrasynaptic sites. Activation of presynaptic A(1) receptors inhibits the release of the majority of transmitters including glutamate, acetylcholine, noradrenaline, 5-HT and dopamine, whilst the stimulation of A(2A) receptors facilitates the release of glutamate and acetylcholine and inhibits the release of GABA. These actions underlie modulation of neuronal excitability, synaptic plasticity and coordination of neural networks and provide intriguing target sites for pharmacological intervention in ischemia and Parkinson's disease. However, despite that adenosine is also released during ischemia, A(1) adenosine receptors do not participate in the modulation of excitotoxic glutamate release, which is nonsynaptic and is due to the reverse operation of transporters. Instead, extrasynaptic A(1) receptors might be responsible for the neuroprotection afforded by A(1) receptor activation.
Figures

Similar articles
-
Adenosine and ATP receptors in the brain.Curr Top Med Chem. 2011;11(8):973-1011. doi: 10.2174/156802611795347627. Curr Top Med Chem. 2011. PMID: 21401499 Review.
-
Adenosine A(2A) receptor facilitation of hippocampal synaptic transmission is dependent on tonic A(1) receptor inhibition.Neuroscience. 2002;112(2):319-29. doi: 10.1016/s0306-4522(02)00080-5. Neuroscience. 2002. PMID: 12044450
-
The role of adenosine in the regulation of sleep.Curr Top Med Chem. 2011;11(8):1047-57. doi: 10.2174/156802611795347654. Curr Top Med Chem. 2011. PMID: 21401496 Review.
-
Adenosine and related drugs in brain diseases: present and future in clinical trials.Curr Top Med Chem. 2011;11(8):1087-101. doi: 10.2174/156802611795347591. Curr Top Med Chem. 2011. PMID: 21401493 Review.
-
GABA release modified by adenosine receptors in mouse hippocampal slices under normal and ischemic conditions.Neurochem Res. 2005 Apr;30(4):467-73. doi: 10.1007/s11064-005-2682-4. Neurochem Res. 2005. PMID: 16076017
Cited by
-
Astrocyte origin of activity-dependent release of ATP and glutamate in hippocampal slices: real-time measurement utilizing microelectrode biosensors.Br J Pharmacol. 2012 Nov;167(5):1000-2. doi: 10.1111/j.1476-5381.2012.02072.x. Br J Pharmacol. 2012. PMID: 22703189 Free PMC article.
-
Homocysteine and A2A-D2 Receptor-Receptor Interaction at Striatal Astrocyte Processes.J Mol Neurosci. 2018 Aug;65(4):456-466. doi: 10.1007/s12031-018-1120-4. Epub 2018 Jul 20. J Mol Neurosci. 2018. PMID: 30030763
-
Hypoxia-ischemia alters nucleotide and nucleoside catabolism and Na+,K+-ATPase activity in the cerebral cortex of newborn rats.Neurochem Res. 2013 Apr;38(4):886-94. doi: 10.1007/s11064-013-0994-3. Epub 2013 Feb 9. Neurochem Res. 2013. PMID: 23397287
-
Current Understanding of the Role of Adenosine Receptors in Cancer.Molecules. 2024 Jul 26;29(15):3501. doi: 10.3390/molecules29153501. Molecules. 2024. PMID: 39124905 Free PMC article. Review.
-
Preclinical Evaluation of the First Adenosine A1 Receptor Partial Agonist Radioligand for Positron Emission Tomography Imaging.J Med Chem. 2018 Nov 21;61(22):9966-9975. doi: 10.1021/acs.jmedchem.8b01009. Epub 2018 Nov 13. J Med Chem. 2018. PMID: 30359014 Free PMC article.
References
-
- Sattin A, Rall T W. The effect of adenosine and adenine nucleotides on the cyclic adenosine 3', 5'-phosphate content of guinea pig cerebral cortex slices. Mol. Pharmacol. 1970;6:13–23. - PubMed
-
- Gustafsson L, Hedqvist P, Fredholm B B, Lundgren G. Inhibition of acetylcholine release in guinea pig ileum by adenosine. Acta Physiol. Scand. 1978;104:469–478. - PubMed
-
- Vizi E S, Knoll J. The inhibitory effect of adenosine and related nucleotides on the release of acetylcholine. Neuroscience. 1976;1:391–398. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

