Decreased leukocyte accumulation and delayed Bordetella pertussis clearance in IL-6-/- mice
- PMID: 21398615
- PMCID: PMC3618952
- DOI: 10.4049/jimmunol.1000594
Decreased leukocyte accumulation and delayed Bordetella pertussis clearance in IL-6-/- mice
Abstract
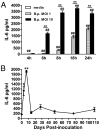
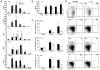
IL-6, a pleiotropic cytokine primarily produced by the innate immune system, has been implicated in the development of acquired immune responses, though its roles are largely undefined and may vary in the context of different diseases. Using a murine model of infection, we established that IL-6 influences the adaptive immune responses against the endemic human respiratory pathogen Bordetella pertussis. IL-6 was induced in the lungs of C57BL/6 mice by B. pertussis. IL-6(-/-) mice showed a protracted infectious course and were less efficiently protected by B. pertussis vaccination than wild-type mice. Abs from IL-6(-/-) mice, though lower in titer, efficiently reduced B. pertussis numbers in IL-6-sufficient mice. Pulmonary leukocyte recruitment and splenic or pulmonary T cell cytokine responses to B. pertussis, including Th1 and Th17 cytokine production, were lower in IL-6(-/-) mice than in wild-type mice. Adoptive transfer of immune wild-type CD4(+) cells ameliorated the defect of IL-6(-/-) mice in the control of B. pertussis numbers. Together, these results reveal the dysregulation of multiple aspects of adaptive immune responses in B. pertussis-infected IL-6(-/-) mice and suggest that IL-6 is involved in regulating Ab generation, pulmonary leukocyte accumulation, and T cell cytokine production in response to B. pertussis as well as the generation of effective vaccine-induced immunity against this pathogen.
Figures

Similar articles
-
Immunological Signatures after Bordetella pertussis Infection Demonstrate Importance of Pulmonary Innate Immune Cells.PLoS One. 2016 Oct 6;11(10):e0164027. doi: 10.1371/journal.pone.0164027. eCollection 2016. PLoS One. 2016. PMID: 27711188 Free PMC article.
-
Azithromycin Clears Bordetella pertussis Infection in Mice but Also Modulates Innate and Adaptive Immune Responses and T Cell Memory.Front Immunol. 2018 Jul 30;9:1764. doi: 10.3389/fimmu.2018.01764. eCollection 2018. Front Immunol. 2018. PMID: 30105030 Free PMC article.
-
Relative contribution of Th1 and Th17 cells in adaptive immunity to Bordetella pertussis: towards the rational design of an improved acellular pertussis vaccine.PLoS Pathog. 2013;9(4):e1003264. doi: 10.1371/journal.ppat.1003264. Epub 2013 Apr 4. PLoS Pathog. 2013. PMID: 23592988 Free PMC article.
-
Functional Programming of Innate Immune Cells in Response to Bordetella pertussis Infection and Vaccination.Adv Exp Med Biol. 2019;1183:53-80. doi: 10.1007/5584_2019_404. Adv Exp Med Biol. 2019. PMID: 31432398 Review.
-
Pertussis: a matter of immune modulation.FEMS Microbiol Rev. 2011 May;35(3):441-74. doi: 10.1111/j.1574-6976.2010.00257.x. Epub 2011 Jan 5. FEMS Microbiol Rev. 2011. PMID: 21204863 Review.
Cited by
-
Immune Response of Indian Preterm Infants to Pentavalent Vaccine Varies With Component Antigens and Gestational Age.Front Immunol. 2021 Apr 23;12:592731. doi: 10.3389/fimmu.2021.592731. eCollection 2021. Front Immunol. 2021. PMID: 33968011 Free PMC article.
-
Evaluation of Adenylate Cyclase Toxoid Antigen in Acellular Pertussis Vaccines by Using a Bordetella pertussis Challenge Model in Mice.Infect Immun. 2018 Sep 21;86(10):e00857-17. doi: 10.1128/IAI.00857-17. Print 2018 Oct. Infect Immun. 2018. PMID: 30012638 Free PMC article.
-
Reinvestigating the Coughing Rat Model of Pertussis To Understand Bordetella pertussis Pathogenesis.Infect Immun. 2021 Nov 16;89(12):e0030421. doi: 10.1128/IAI.00304-21. Epub 2021 Jun 14. Infect Immun. 2021. PMID: 34125597 Free PMC article.
-
Review of the neutrophil response to Bordetella pertussis infection.Pathog Dis. 2015 Dec;73(9):ftv081. doi: 10.1093/femspd/ftv081. Epub 2015 Oct 2. Pathog Dis. 2015. PMID: 26432818 Free PMC article. Review.
-
Caspase-1-independent interleukin-1β is required for clearance of Bordetella pertussis infections and whole-cell vaccine-mediated immunity.PLoS One. 2014 Sep 8;9(9):e107188. doi: 10.1371/journal.pone.0107188. eCollection 2014. PLoS One. 2014. PMID: 25198773 Free PMC article.
References
-
- Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol. 2005;175:3463–3468. - PubMed
-
- Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. - PubMed
-
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

