Disruption of the transient receptor potential vanilloid 1 can affect survival, bacterial clearance, and cytokine gene expression during murine sepsis
- PMID: 21383614
- PMCID: PMC3119195
- DOI: 10.1097/ALN.0b013e318212515b
Disruption of the transient receptor potential vanilloid 1 can affect survival, bacterial clearance, and cytokine gene expression during murine sepsis
Abstract
Background: Previous studies suggest that the transient receptor potential vanilloid 1 (TRPV1) channel has a role in sepsis, but it is unclear whether its effect on survival and immune response is beneficial or harmful.
Methods: We studied the effects of genetic (Trpv1-knockout vs. wild-type [WT] mice) and pharmacologic disruption of TRPV1 with resiniferatoxin (an agonist) or capsazepine (an antagonist) on mortality, bacterial clearance, and cytokine expression during lipopolysaccharide or cecal ligation and puncture-induced sepsis.
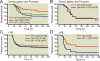
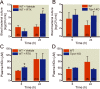
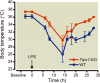
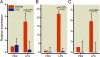
Results: After cecal ligation and puncture, genetic disruption of TRPV1 in Trpv1-knockout versus WT mice was associated with increased mortality risk (hazard ratio, 2.17; 95% CI, 1.23-3.81; P = 0.01). Furthermore, pharmacologic disruption of TRPV1 with intrathecal resiniferatoxin, compared with vehicle, increased mortality risk (hazard ratio, 1.80; 95% CI, 1.05-3.2; P = 0.03) in WT, but not in Trpv1-knockout, mice. After lipopolysaccharide, neither genetic (Trpv1 knockout) nor pharmacologic disruption of TRPV1 with resiniferatoxin had significant effect on survival compared with respective controls. In contrast, after lipopolysaccharide, pharmacologic disruption of TRPV1 with capsazepine, compared with vehicle, increased mortality risk (hazard ratio, 1.92; 95% CI, 1.02-3.61; P = 0.04) in WT animals. Furthermore, after cecal ligation and puncture, increased mortality in resiniferatoxin-treated WT animals was associated with higher blood bacterial count (P = 0.0004) and higher nitrate/nitrite concentrations and down-regulation of tumor necrosis factor α expression (P = 0.004) compared with controls.
Conclusions: Genetic or pharmacologic disruption of TRPV1 can affect mortality, blood bacteria clearance, and cytokine response in sepsis in patterns that may vary according to the sepsis-inducing event and the method of TRPV1 disruption.
Figures

Similar articles
-
Aging reverses the role of the transient receptor potential vanilloid-1 channel in systemic inflammation from anti-inflammatory to proinflammatory.Cell Cycle. 2012 Jan 15;11(2):343-9. doi: 10.4161/cc.11.2.18772. Epub 2012 Jan 15. Cell Cycle. 2012. PMID: 22214765 Free PMC article.
-
Transient receptor potential melastatin 2 protects mice against polymicrobial sepsis by enhancing bacterial clearance.Anesthesiology. 2014 Aug;121(2):336-51. doi: 10.1097/ALN.0000000000000275. Anesthesiology. 2014. PMID: 24781495
-
α7 Nicotinic acetylcholine receptor contributes to the alleviation of lung ischemia-reperfusion injury by transient receptor potential vanilloid type 1 stimulation.J Surg Res. 2018 Oct;230:164-174. doi: 10.1016/j.jss.2018.05.038. Epub 2018 Jun 23. J Surg Res. 2018. PMID: 30100034
-
Targeting TRPV1 and TRPV2 for potential therapeutic interventions in cardiovascular disease.Transl Res. 2013 Jun;161(6):469-76. doi: 10.1016/j.trsl.2013.02.003. Epub 2013 Feb 27. Transl Res. 2013. PMID: 23453732 Review.
-
TRPV1 and SP: key elements for sepsis outcome?Br J Pharmacol. 2013 Dec;170(7):1279-92. doi: 10.1111/bph.12056. Br J Pharmacol. 2013. PMID: 23145480 Free PMC article. Review.
Cited by
-
Capsaicin, The Vanilloid Receptor TRPV1 Agonist in Neuroprotection: Mechanisms Involved and Significance.Neurochem Res. 2023 Nov;48(11):3296-3315. doi: 10.1007/s11064-023-03983-z. Epub 2023 Jul 26. Neurochem Res. 2023. PMID: 37493882 Free PMC article. Review.
-
N-Arachidonoyl Dopamine Modulates Acute Systemic Inflammation via Nonhematopoietic TRPV1.J Immunol. 2017 Aug 15;199(4):1465-1475. doi: 10.4049/jimmunol.1602151. Epub 2017 Jul 12. J Immunol. 2017. PMID: 28701511 Free PMC article.
-
TRPV1 Contributes to Cerebral Malaria Severity and Mortality by Regulating Brain Inflammation.Oxid Med Cell Longev. 2019 May 16;2019:9451671. doi: 10.1155/2019/9451671. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31223430 Free PMC article.
-
Involvement of the TRPV1 channel in the modulation of spontaneous locomotor activity, physical performance and physical exercise-induced physiological responses.Braz J Med Biol Res. 2016;49(6):e5183. doi: 10.1590/1414-431X20165183. Epub 2016 May 10. Braz J Med Biol Res. 2016. PMID: 27191606 Free PMC article. Review.
-
Transient Receptor Potential Channels and Auditory Functions.Antioxid Redox Signal. 2022 Jun;36(16-18):1158-1170. doi: 10.1089/ars.2021.0191. Epub 2021 Dec 31. Antioxid Redox Signal. 2022. PMID: 34465184 Free PMC article. Review.
References
-
- Okajima K, Harada N. Regulation of inflammatory responses by sensory neurons: Molecular mechanism(s) and possible therapeutic applications. Curr Med Chem. 2006;13:2241–51. - PubMed
-
- Alawi K, Keeble J. The paradoxical role of the transient receptor potential vanilloid 1 receptor in inflammation. Pharmacol Ther. 2010;125:181–95. - PubMed
-
- Richardson JD, Vasko MR. Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther. 2002;302:839–45. - PubMed
-
- Tender GC, Walbridge S, Olah Z, Karai L, Iadarola M, Oldfield EH, Lonser RR. Selective ablation of nociceptive neurons for elimination of hyperalgesia and neurogenic inflammation. J Neurosurg. 2005;102:522–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

