Herpesvirus telomeric repeats facilitate genomic integration into host telomeres and mobilization of viral DNA during reactivation
- PMID: 21383055
- PMCID: PMC3058580
- DOI: 10.1084/jem.20101402
Herpesvirus telomeric repeats facilitate genomic integration into host telomeres and mobilization of viral DNA during reactivation
Abstract
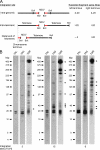
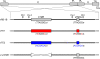
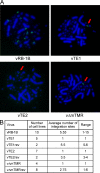
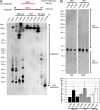
Some herpesviruses, particularly lymphotropic viruses such as Marek's disease virus (MDV) and human herpesvirus 6 (HHV-6), integrate their DNA into host chromosomes. MDV and HHV-6, among other herpesviruses, harbor telomeric repeats (TMRs) identical to host telomeres at either end of their linear genomes. Using MDV as a natural virus-host model, we show that herpesvirus TMRs facilitate viral genome integration into host telomeres and that integration is important for establishment of latency and lymphoma formation. Integration into host telomeres also aids in reactivation from the quiescent state of infection. Our results and the presence of TMRs in many herpesviruses suggest that integration mediated by viral TMRs is a conserved mechanism, which ensures faithful virus genome maintenance in host cells during cell division and allows efficient mobilization of dormant viral genomes. This finding is of particular importance as reactivation is critical for virus spread between susceptible individuals and is necessary for continued herpesvirus evolution and survival.
Figures


Similar articles
-
Impact of viral telomeric repeat sequences on herpesvirus vector vaccine integration and persistence.PLoS Pathog. 2024 May 28;20(5):e1012261. doi: 10.1371/journal.ppat.1012261. eCollection 2024 May. PLoS Pathog. 2024. PMID: 38805555 Free PMC article.
-
Herpesvirus Genome Integration into Telomeric Repeats of Host Cell Chromosomes.Annu Rev Virol. 2014 Nov;1(1):215-35. doi: 10.1146/annurev-virology-031413-085422. Epub 2014 Jun 27. Annu Rev Virol. 2014. PMID: 26958721
-
Role of the short telomeric repeat region in Marek's disease virus replication, genomic integration, and lymphomagenesis.J Virol. 2014 Dec;88(24):14138-47. doi: 10.1128/JVI.02437-14. Epub 2014 Oct 1. J Virol. 2014. PMID: 25275118 Free PMC article.
-
Telomeres and Telomerase: Role in Marek's Disease Virus Pathogenesis, Integration and Tumorigenesis.Viruses. 2017 Jul 4;9(7):173. doi: 10.3390/v9070173. Viruses. 2017. PMID: 28677643 Free PMC article. Review.
-
Latency, Integration, and Reactivation of Human Herpesvirus-6.Viruses. 2017 Jul 24;9(7):194. doi: 10.3390/v9070194. Viruses. 2017. PMID: 28737715 Free PMC article. Review.
Cited by
-
Telomeric repeats in the commercial SB-1 vaccine facilitate viral integration and contribute to vaccine efficacy.NPJ Vaccines. 2024 Aug 21;9(1):154. doi: 10.1038/s41541-024-00945-6. NPJ Vaccines. 2024. PMID: 39169010 Free PMC article.
-
Horizontal gene transfer events reshape the global landscape of arm race between viruses and homo sapiens.Sci Rep. 2016 Jun 7;6:26934. doi: 10.1038/srep26934. Sci Rep. 2016. PMID: 27270140 Free PMC article.
-
Hypoxia and HIF-1 Trigger Marek's Disease Virus Reactivation in Lymphoma-Derived Latently Infected T Lymphocytes.J Virol. 2022 Mar 9;96(5):e0142721. doi: 10.1128/JVI.01427-21. Epub 2021 Dec 22. J Virol. 2022. PMID: 34936483 Free PMC article.
-
Molecular characterization of herpes simplex virus 2 strains by analysis of microsatellite polymorphism.J Clin Microbiol. 2013 Nov;51(11):3616-23. doi: 10.1128/JCM.01714-13. Epub 2013 Aug 21. J Clin Microbiol. 2013. PMID: 23966512 Free PMC article.
-
Overexpression of cellular telomerase RNA enhances virus-induced cancer formation.Oncogene. 2019 Mar;38(10):1778-1786. doi: 10.1038/s41388-018-0544-1. Epub 2018 Oct 19. Oncogene. 2019. PMID: 30846849
References
-
- Arbuckle J.H., Medveczky M.M., Luka J., Hadley S.H., Luegmayr A., Ablashi D., Lund T.C., Tolar J., De Meirleir K., Montoya J.G., et al. 2010. The latent human herpesvirus-6A genome specifically integrates in telomeres of human chromosomes in vivo and in vitro. Proc. Natl. Acad. Sci. USA. 107:5563–5568 10.1073/pnas.0913586107 - DOI - PMC - PubMed
-
- Brown A.C., Baigent S.J., Smith L.P., Chattoo J.P., Petherbridge L.J., Hawes P., Allday M.J., Nair V. 2006. Interaction of MEQ protein and C-terminal-binding protein is critical for induction of lymphomas by Marek’s disease virus. Proc. Natl. Acad. Sci. USA. 103:1687–1692 10.1073/pnas.0507595103 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

