Goodpasture's disease: molecular architecture of the autoantigen provides clues to etiology and pathogenesis
- PMID: 21378566
- PMCID: PMC3159420
- DOI: 10.1097/MNH.0b013e328344ff20
Goodpasture's disease: molecular architecture of the autoantigen provides clues to etiology and pathogenesis
Abstract
Purpose of review: Goodpasture's disease is an autoimmune disorder characterized by the deposition of pathogenic autoantibodies in basement membranes of kidney and lung, which induces rapidly progressive glomerulonephritis and pulmonary hemorrhage. The target antigen is the α3NC1 domain of collagen IV, which is expressed in target organs as an α345 network. Recent studies of specificity and epitopes of Goodpasture's autoantibodies and discovery of novel posttranslational modification of the antigen, a sulfilimine bond, provide further insight into mechanisms of initiation and progression of Goodpasture's disease.
Recent findings: Analysis of the specificity of Goodpasture's autoantibodies revealed a distinct subset of circulating and kidney-bound antiα5NC1 antibody, which is associated with loss of kidney function. Structural integrity of the α345NC1 hexamer is stabilized by the novel sulfilimine crosslinks conferring immune privilege to the Goodpasture's autoantigen. Native antibodies may contribute to establishment of immune tolerance to autoantigen. Structural analysis of epitopes for autoantibodies and alloantibodies indicates a critical role of conformational change in the α345NC1 hexamer in eliciting an autoimmune response in Goodpasture's disease.
Summary: Understanding of the quaternary structure of the Goodpasture's autoantigen continues to provide insights into autoimmune mechanisms that serve as a basis for development of novel diagnostic tools and therapies for Goodpasture's disease.
Figures
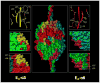
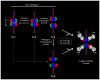
Similar articles
-
Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis.N Engl J Med. 2010 Jul 22;363(4):343-54. doi: 10.1056/NEJMoa0910500. N Engl J Med. 2010. PMID: 20660402 Free PMC article.
-
Goodpasture's autoimmune disease - A collagen IV disorder.Matrix Biol. 2018 Oct;71-72:240-249. doi: 10.1016/j.matbio.2018.05.004. Epub 2018 May 12. Matrix Biol. 2018. PMID: 29763670 Free PMC article. Review.
-
Molecular Analysis of Goodpasture's Disease Following Hematopoietic Stem Cell Transplant in a Pediatric Patient, Recalls the Conformeropathy of Wild-Type Anti-GBM Disease.Front Immunol. 2019 Nov 14;10:2659. doi: 10.3389/fimmu.2019.02659. eCollection 2019. Front Immunol. 2019. PMID: 31798588 Free PMC article.
-
Antibodies to α5 chain of collagen IV are pathogenic in Goodpasture's disease.J Autoimmun. 2016 Jun;70:1-11. doi: 10.1016/j.jaut.2016.04.001. Epub 2016 Apr 23. J Autoimmun. 2016. PMID: 27117167 Free PMC article.
-
Cutting edge issues in Goodpasture's disease.Clin Rev Allergy Immunol. 2011 Oct;41(2):151-62. doi: 10.1007/s12016-010-8222-2. Clin Rev Allergy Immunol. 2011. PMID: 21207195 Review.
Cited by
-
Frequently relapsing anti-glomerular basement membrane antibody disease with changing clinical phenotype and antibody characteristics over time.Clin Kidney J. 2016 Oct;9(5):661-4. doi: 10.1093/ckj/sfw048. Epub 2016 Jun 19. Clin Kidney J. 2016. PMID: 27679711 Free PMC article.
-
Coexistence of Anti-Glomerular Basement Membrane Antibodies and Anti-Neutrophil Cytoplasmic Antibodies in a Child With Human Leukocyte Antigen Susceptibility and Detailed Antibody Description: A Case Report.Medicine (Baltimore). 2015 Jul;94(29):e1179. doi: 10.1097/MD.0000000000001179. Medicine (Baltimore). 2015. PMID: 26200622 Free PMC article.
-
Pathogenesis of membranous nephropathy: recent advances and future challenges.Nat Rev Nephrol. 2012 Feb 28;8(4):203-13. doi: 10.1038/nrneph.2012.35. Nat Rev Nephrol. 2012. PMID: 22371247 Review.
-
Autoantigenesis: the evolution of protein modifications in autoimmune disease.Curr Opin Immunol. 2012 Feb;24(1):112-8. doi: 10.1016/j.coi.2011.12.003. Epub 2011 Dec 29. Curr Opin Immunol. 2012. PMID: 22209691 Free PMC article. Review.
-
Optimizing the translational value of animal models of glomerulonephritis: insights from recent murine prototypes.Am J Physiol Renal Physiol. 2016 Sep 1;311(3):F487-95. doi: 10.1152/ajprenal.00275.2016. Epub 2016 Jun 22. Am J Physiol Renal Physiol. 2016. PMID: 27335377 Free PMC article. Review.
References
-
- Salama AD, Levy JB, Lightstone L, Pusey CD. Goodpasture's disease. Lancet. 2001;358:917–20. - PubMed
-
- Wilson CB, Dixon FJ. Anti-glomerular basement membrane antibody-induced glomerulonephritis. Kidney Int. 1973;3:74–89. - PubMed
-
- Lockwood CM, Rees AJ, Pearson TA, et al. Immunosuppression and plasma-exchange in the treatment of Goodpasture's syndrome. Lancet. 1976;1:711–5. - PubMed
-
- Butkowski RJ, Langeveld JP, Wieslander J, et al. Localization of the Goodpasture epitope to a novel chain of basement membrane collagen. J Biol Chem. 1987;262:7874–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

