Partial efficacy of a VSV-SIV/MVA-SIV vaccine regimen against oral SIV challenge in infant macaques
- PMID: 21377510
- PMCID: PMC3078946
- DOI: 10.1016/j.vaccine.2011.02.051
Partial efficacy of a VSV-SIV/MVA-SIV vaccine regimen against oral SIV challenge in infant macaques
Abstract
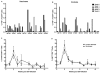
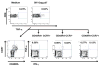
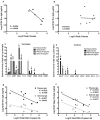
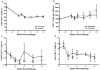
Despite antiretroviral medications, the rate of pediatric HIV-1 infections through breast-milk transmission has been staggering in developing countries. Therefore, the development of a vaccine to protect vulnerable infant populations should be actively pursued. We previously demonstrated that oral immunization of newborn macaques with vesicular stomatitis virus expressing simian immunodeficiency virus genes (VSV-SIV) followed 2 weeks later by an intramuscular boost with modified vaccinia ankara virus expressing SIV (MVA-SIV) successfully induced SIV-specific T and B cell responses in multiple lymphoid tissues, including the tonsil and intestine [13]. In the current study, we tested the oral VSV-SIV prime/systemic MVA-SIV boost vaccine for efficacy against multiple oral SIVmac251 challenges starting two weeks after the booster vaccination. The vaccine did not prevent SIV infection. However, in vaccinated infants, the level of SIV-specific plasma IgA (but not IgG) at the time of challenge was inversely correlated with peak viremia. In addition, the levels of SIV-specific IgA in saliva and plasma were inversely correlated with viral load at euthanasia. Animals with tonsils that contained higher frequencies of SIV-specific TNF-α- or IFN-γ-producing CD8(+) T cells and central memory T cells at euthanasia also had lower viremia. Interestingly, a marked depletion of CD25(+)FoxP3(+)CD4(+) T cells was observed in the tonsils as well as the intestine of these animals, implying that T regulatory cells may be a major target of SIV infection in infant macaques. Overall, the data suggest that, in infant macaques orally infected with SIV, the co-induction of local antiviral cytotoxic T cells and T regulatory cells that promote the development of IgA responses may result in better control of viral replication. Thus, future vaccination efforts should be directed towards induction of IgA and mucosal T cell responses to prevent or reduce virus replication in infants.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Vaccine-Elicited Mucosal and Systemic Antibody Responses Are Associated with Reduced Simian Immunodeficiency Viremia in Infant Rhesus Macaques.J Virol. 2016 Jul 27;90(16):7285-7302. doi: 10.1128/JVI.00481-16. Print 2016 Aug 15. J Virol. 2016. PMID: 27252535 Free PMC article.
-
Immunogenicity of viral vector, prime-boost SIV vaccine regimens in infant rhesus macaques: attenuated vesicular stomatitis virus (VSV) and modified vaccinia Ankara (MVA) recombinant SIV vaccines compared to live-attenuated SIV.Vaccine. 2010 Feb 10;28(6):1481-92. doi: 10.1016/j.vaccine.2009.11.061. Epub 2009 Dec 6. Vaccine. 2010. PMID: 19995539 Free PMC article.
-
Oral Coadministration of an Intramuscular DNA/Modified Vaccinia Ankara Vaccine for Simian Immunodeficiency Virus Is Associated with Better Control of Infection in Orally Exposed Infant Macaques.AIDS Res Hum Retroviruses. 2019 Mar;35(3):310-325. doi: 10.1089/AID.2018.0180. Epub 2018 Nov 27. AIDS Res Hum Retroviruses. 2019. PMID: 30303405 Free PMC article.
-
Antiviral CD8+ T cells in the genital tract control viral replication and delay progression to AIDS after vaginal SIV challenge in rhesus macaques immunized with virulence attenuated SHIV 89.6.J Intern Med. 2009 Jan;265(1):67-77. doi: 10.1111/j.1365-2796.2008.02051.x. J Intern Med. 2009. PMID: 19093961 Free PMC article. Review.
-
Host range restricted, non-replicating vaccinia virus vectors as vaccine candidates.Adv Exp Med Biol. 1996;397:7-13. doi: 10.1007/978-1-4899-1382-1_2. Adv Exp Med Biol. 1996. PMID: 8718576 Free PMC article. Review.
Cited by
-
Vaccine-Elicited Mucosal and Systemic Antibody Responses Are Associated with Reduced Simian Immunodeficiency Viremia in Infant Rhesus Macaques.J Virol. 2016 Jul 27;90(16):7285-7302. doi: 10.1128/JVI.00481-16. Print 2016 Aug 15. J Virol. 2016. PMID: 27252535 Free PMC article.
-
Simian-Human Immunodeficiency Virus SHIV.CH505-Infected Infant and Adult Rhesus Macaques Exhibit Similar Env-Specific Antibody Kinetics, despite Distinct T-Follicular Helper and Germinal Center B Cell Landscapes.J Virol. 2019 Jul 17;93(15):e00168-19. doi: 10.1128/JVI.00168-19. Print 2019 Aug 1. J Virol. 2019. PMID: 31092583 Free PMC article.
-
The oral mucosa immune environment and oral transmission of HIV/SIV.Immunol Rev. 2013 Jul;254(1):34-53. doi: 10.1111/imr.12078. Immunol Rev. 2013. PMID: 23772613 Free PMC article. Review.
-
A vaccine against CCR5 protects a subset of macaques upon intravaginal challenge with simian immunodeficiency virus SIVmac251.J Virol. 2014 Feb;88(4):2011-24. doi: 10.1128/JVI.02447-13. Epub 2013 Dec 4. J Virol. 2014. PMID: 24307581 Free PMC article.
-
Impact of Poxvirus Vector Priming, Protein Coadministration, and Vaccine Intervals on HIV gp120 Vaccine-Elicited Antibody Magnitude and Function in Infant Macaques.Clin Vaccine Immunol. 2017 Oct 5;24(10):e00231-17. doi: 10.1128/CVI.00231-17. Print 2017 Oct. Clin Vaccine Immunol. 2017. PMID: 28814388 Free PMC article.
References
-
- Mofenson LM. Antiretroviral drugs to prevent breastfeeding HIV transmission. Antivir Ther. 15(4):537–53. - PubMed
-
- Mofenson LM. Protecting the next generation--eliminating perinatal HIV-1 infection. N Engl J Med. Jun 17;362(24):2316–8. - PubMed
-
- Zolfo M, De Weggheleire A, Schouten E, Lynen L. Time for “test and treat” in prevention of mother-to-child transmission programs in low- and middle-income countries. J Acquir Immune Defic Syndr. Nov 1;55(3):287–9. - PubMed
-
- Marazzi MC, Liotta G, Nielsen-Saines K, Haswell J, Magid NA, Buonomo E, et al. Extended antenatal antiretroviral use correlates with improved infant outcomes throughout the first year of life. AIDS. Nov 27;24(18):2819–26. - PubMed
-
- van der Horst C, Chasela C, Ahmed Y, Hoffman I, Hosseinipour M, Knight R, et al. Modifications of a large HIV prevention clinical trial to fit changing realities: a case study of the Breastfeeding, Antiretroviral, and Nutrition (BAN) protocol in Lilongwe, Malawi. Contemp Clin Trials. 2009 Jan;30(1):24–33. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

