Dimeric states of neural- and epithelial-cadherins are distinguished by the rate of disassembly
- PMID: 21375242
- PMCID: PMC3471160
- DOI: 10.1021/bi2001246
Dimeric states of neural- and epithelial-cadherins are distinguished by the rate of disassembly
Abstract
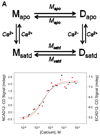
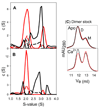
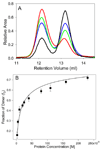
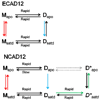
Epithelial- and neural-cadherins are specifically localized at synapses in neurons which can change the shape and contact surface on a time scale of seconds to months. We have focused our studies on the role of the extracellular domains of cadherins in the dynamics of synapses. The kinetics of dimer disassembly of the first two extracellular domains of E- and N-cadherin, ECAD12 and NCAD12, were studied with analytical size exclusion chromatography and sedimentation velocity. NCAD12 forms three different dimers that are distinguished by assembly conditions and kinetics of dissociation. ECAD12 dimer disassembles rapidly regardless of the calcium concentration, whereas the disassembly of NCAD12 dimers was strongly dependent on calcium concentration. In addition to the apo- and saturated-dimeric forms of NCAD12, there is a third dimeric form that is a slow exchange dimer. This third dimeric form for NCAD12, formed by decalcification of the calcium-saturated dimer, was kinetically trapped in apo-conditions and did not disassemble over a period of months. Sedimentation velocity experiments showed that this dimer, upon addition of calcium, had similar weighted averages as a calcium-saturated dimer. These studies provide evidence that the kinetics of dimer disassembly of the extracellular domains may be a major contributor to the morphological dynamics of synapses in vivo.
Figures


Similar articles
-
X-interface is not the explanation for the slow disassembly of N-cadherin dimers in the apo state.Protein Sci. 2012 Jul;21(7):1006-14. doi: 10.1002/pro.2083. Epub 2012 May 24. Protein Sci. 2012. PMID: 22544613 Free PMC article.
-
Sequential binding of calcium leads to dimerization in neural cadherin.Biochemistry. 2011 Apr 12;50(14):2973-82. doi: 10.1021/bi101872b. Epub 2011 Mar 21. Biochemistry. 2011. PMID: 21366346
-
Basic residue at position 14 is not required for fast assembly and disassembly kinetics in neural cadherin.Biochemistry. 2015 Jan 27;54(3):836-43. doi: 10.1021/bi5010415. Epub 2015 Jan 8. Biochemistry. 2015. PMID: 25517179
-
Calcium-dependent homoassociation of E-cadherin by NMR spectroscopy: changes in mobility, conformation and mapping of contact regions.J Mol Biol. 2002 Dec 6;324(4):823-39. doi: 10.1016/s0022-2836(02)01137-3. J Mol Biol. 2002. PMID: 12460580
-
Thinking outside the cell: how cadherins drive adhesion.Trends Cell Biol. 2012 Jun;22(6):299-310. doi: 10.1016/j.tcb.2012.03.004. Epub 2012 May 1. Trends Cell Biol. 2012. PMID: 22555008 Free PMC article. Review.
Cited by
-
Adherens junction assembly.Subcell Biochem. 2012;60:89-108. doi: 10.1007/978-94-007-4186-7_5. Subcell Biochem. 2012. PMID: 22674069 Free PMC article. Review.
-
X-interface is not the explanation for the slow disassembly of N-cadherin dimers in the apo state.Protein Sci. 2012 Jul;21(7):1006-14. doi: 10.1002/pro.2083. Epub 2012 May 24. Protein Sci. 2012. PMID: 22544613 Free PMC article.
-
Ideal, catch, and slip bonds in cadherin adhesion.Proc Natl Acad Sci U S A. 2012 Nov 13;109(46):18815-20. doi: 10.1073/pnas.1208349109. Epub 2012 Oct 29. Proc Natl Acad Sci U S A. 2012. PMID: 23112161 Free PMC article.
References
-
- Shapiro L, Love J, Colman DR. Adhesion molecules in the nervous system: structural insights into function and diversity. Annu Rev Neurosci. 2007;30:451–474. - PubMed
-
- Yu X, Malenka RC. Multiple functions for the cadherin/catenin complex during neuronal development. Neuropharmacology. 2004;47:779–786. - PubMed
-
- Huntley GW, Gil O, Bozdagi O. The cadherin family of cell adhesion molecules: multiple roles in synaptic plasticity. Neuroscientist. 2002;8:221–233. - PubMed
-
- Bekirov IH, Needleman LA, Zhang W, Benson DL. Identification and localization of multiple classic cadherins in developing rat limbic system. Neuroscience. 2002;115:213–227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

