Vascular remodeling and arterial calcification are directly mediated by S100A12 (EN-RAGE) in chronic kidney disease
- PMID: 21372560
- PMCID: PMC3064943
- DOI: 10.1159/000324693
Vascular remodeling and arterial calcification are directly mediated by S100A12 (EN-RAGE) in chronic kidney disease
Abstract
Background: The proinflammatory cytokine S100A12 (also known as EN-RAGE) is associated with cardiovascular morbidity and mortality in hemodialysis patients. In the current study, we tested the hypothesis that S100A12 expressed in vascular smooth muscle in nonatherosclerosis-prone C57BL/6J mice on normal rodent chow diet, but exposed to the metabolic changes of chronic kidney disease (CKD), would develop vascular disease resembling that observed in patients with CKD.
Methods: CKD was induced in S100A12 transgenic mice and wild-type littermate mice not expressing human S100A12 by surgical ligation of the ureters. The aorta was analyzed after 7 weeks of elevated BUN (blood urea nitrogen), and cultured aortic smooth muscle cells were studied.
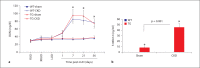
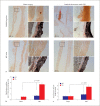
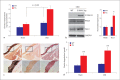
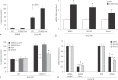
Results: We found enhanced vascular medial calcification in S100A12tg mice subjected to CKD. Vascular calcification was mediated, at least in part, by activation of the receptor for S100A12, RAGE (receptor for advanced glycation endproducts), and by enhanced oxidative stress, since inhibition of NADPH-oxidase Nox1 and limited access of S100A12 to RAGE attenuated the calcification and gene expression of osteoblastic genes in cultured vascular smooth muscle cells.
Conclusion: S100A12 augments CKD-triggered osteogenesis in murine vasculature, reminiscent of features associated with enhanced vascular calcification in patients with chronic and end-stage kidney disease.
Copyright © 2011 S. Karger AG, Basel.
Figures
Similar articles
-
S100A12 expression in thoracic aortic aneurysm is associated with increased risk of dissection and perioperative complications.J Am Coll Cardiol. 2012 Aug 21;60(8):775-85. doi: 10.1016/j.jacc.2012.04.027. Epub 2012 Jul 18. J Am Coll Cardiol. 2012. PMID: 22818064 Free PMC article.
-
S100A12 in vascular smooth muscle accelerates vascular calcification in apolipoprotein E-null mice by activating an osteogenic gene regulatory program.Arterioscler Thromb Vasc Biol. 2011 Feb;31(2):337-44. doi: 10.1161/ATVBAHA.110.217745. Epub 2010 Oct 21. Arterioscler Thromb Vasc Biol. 2011. PMID: 20966394 Free PMC article.
-
S100/Calgranulin-mediated inflammation accelerates left ventricular hypertrophy and aortic valve sclerosis in chronic kidney disease in a receptor for advanced glycation end products-dependent manner.Arterioscler Thromb Vasc Biol. 2014 Jul;34(7):1399-411. doi: 10.1161/ATVBAHA.114.303508. Epub 2014 May 22. Arterioscler Thromb Vasc Biol. 2014. PMID: 24855059 Free PMC article.
-
S100A12 and the S100/Calgranulins: Emerging Biomarkers for Atherosclerosis and Possibly Therapeutic Targets.Arterioscler Thromb Vasc Biol. 2015 Dec;35(12):2496-507. doi: 10.1161/ATVBAHA.115.302072. Epub 2015 Oct 29. Arterioscler Thromb Vasc Biol. 2015. PMID: 26515415 Free PMC article. Review.
-
S100 proteins in atherosclerosis.Clin Chim Acta. 2020 Mar;502:293-304. doi: 10.1016/j.cca.2019.11.019. Epub 2019 Nov 30. Clin Chim Acta. 2020. PMID: 31794767 Review.
Cited by
-
Regulation of Vascular Calcification by Reactive Oxygen Species.Antioxidants (Basel). 2020 Oct 8;9(10):963. doi: 10.3390/antiox9100963. Antioxidants (Basel). 2020. PMID: 33049989 Free PMC article. Review.
-
Plasma levels of advanced glycation endproducts are associated with type 1 diabetes and coronary artery calcification.Cardiovasc Diabetol. 2013 Oct 17;12:149. doi: 10.1186/1475-2840-12-149. Cardiovasc Diabetol. 2013. PMID: 24134530 Free PMC article.
-
Circulating S100A12 Levels Are Associated with Progression of Abdominal Aortic Calcification in Hemodialysis Patients.PLoS One. 2016 Feb 25;11(2):e0150145. doi: 10.1371/journal.pone.0150145. eCollection 2016. PLoS One. 2016. PMID: 26914918 Free PMC article.
-
Molecular and cellular aspects of calcific aortic valve disease.Circ Res. 2013 Jul 5;113(2):198-208. doi: 10.1161/CIRCRESAHA.113.300155. Circ Res. 2013. PMID: 23833294 Free PMC article. Review.
-
S100A12 expression in thoracic aortic aneurysm is associated with increased risk of dissection and perioperative complications.J Am Coll Cardiol. 2012 Aug 21;60(8):775-85. doi: 10.1016/j.jacc.2012.04.027. Epub 2012 Jul 18. J Am Coll Cardiol. 2012. PMID: 22818064 Free PMC article.
References
-
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. - PubMed
-
- Mori Y, Kosaki A, Kishimoto N, Kimura T, Iida K, Fukui M, Nakajima F, Nagahara M, Urakami M, Iwasaka T, Matsubara H. Increased plasma S100A12 (EN-RAGE) levels in hemodialysis patients with atherosclerosis. Am J Nephrol. 2009;29:18–24. - PubMed
-
- Nakashima A, Carrero JJ, Qureshi AR, Miyamoto T, Anderstam B, Barany P, Heimburger O, Stenvinkel P, Lindholm B. Effect of circulating soluble receptor for advanced glycation end products (sRAGE) and the proinflammatory RAGE ligand (EN-RAGE, S100A12) on mortality in hemodialysis patients. Clin J Am Soc Nephrol. 2010;12:2213–2219. - PMC - PubMed
-
- Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. - PubMed
-
- Leclerc E, Fritz G, Vetter SW, Heizmann CW. Binding of S100 proteins to RAGE: an update. Biochim Biophys Acta. 2009;1793:993–1007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

