Uncoordinated transcription and compromised muscle function in the lmna-null mouse model of Emery- Emery-Dreyfuss muscular dystrophy
- PMID: 21364987
- PMCID: PMC3043058
- DOI: 10.1371/journal.pone.0016651
Uncoordinated transcription and compromised muscle function in the lmna-null mouse model of Emery- Emery-Dreyfuss muscular dystrophy
Abstract
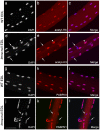
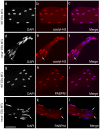
LMNA encodes both lamin A and C: major components of the nuclear lamina. Mutations in LMNA underlie a range of tissue-specific degenerative diseases, including those that affect skeletal muscle, such as autosomal-Emery-Dreifuss muscular dystrophy (A-EDMD) and limb girdle muscular dystrophy 1B. Here, we examine the morphology and transcriptional activity of myonuclei, the structure of the myotendinous junction and the muscle contraction dynamics in the lmna-null mouse model of A-EDMD. We found that there were fewer myonuclei in lmna-null mice, of which ∼50% had morphological abnormalities. Assaying transcriptional activity by examining acetylated histone H3 and PABPN1 levels indicated that there was a lack of coordinated transcription between myonuclei lacking lamin A/C. Myonuclei with abnormal morphology and transcriptional activity were distributed along the length of the myofibre, but accumulated at the myotendinous junction. Indeed, in addition to the presence of abnormal myonuclei, the structure of the myotendinous junction was perturbed, with disorganised sarcomeres and reduced interdigitation with the tendon, together with lipid and collagen deposition. Functionally, muscle contraction became severely affected within weeks of birth, with specific force generation dropping as low as ∼65% and ∼27% of control values in the extensor digitorum longus and soleus muscles respectively. These observations illustrate the importance of lamin A/C for correct myonuclear function, which likely acts synergistically with myotendinous junction disorganisation in the development of A-EDMD, and the consequential reduction in force generation and muscle wasting.
Conflict of interest statement
Figures




Similar articles
-
Lamin A/C-mediated neuromuscular junction defects in Emery-Dreifuss muscular dystrophy.J Cell Biol. 2009 Jan 12;184(1):31-44. doi: 10.1083/jcb.200811035. Epub 2009 Jan 5. J Cell Biol. 2009. PMID: 19124654 Free PMC article.
-
Nuclear changes in skeletal muscle extend to satellite cells in autosomal dominant Emery-Dreifuss muscular dystrophy/limb-girdle muscular dystrophy 1B.Neuromuscul Disord. 2009 Jan;19(1):29-36. doi: 10.1016/j.nmd.2008.09.018. Epub 2008 Dec 12. Neuromuscul Disord. 2009. PMID: 19070492
-
Lamin A/C Assembly Defects in LMNA-Congenital Muscular Dystrophy Is Responsible for the Increased Severity of the Disease Compared with Emery-Dreifuss Muscular Dystrophy.Cells. 2020 Mar 31;9(4):844. doi: 10.3390/cells9040844. Cells. 2020. PMID: 32244403 Free PMC article.
-
Does satellite cell dysfunction contribute to disease progression in Emery-Dreifuss muscular dystrophy?Biochem Soc Trans. 2008 Dec;36(Pt 6):1344-9. doi: 10.1042/BST0361344. Biochem Soc Trans. 2008. PMID: 19021553 Review.
-
Clinical aspects of Emery-Dreifuss muscular dystrophy.Nucleus. 2018 Jan 1;9(1):268-274. doi: 10.1080/19491034.2018.1462635. Nucleus. 2018. PMID: 29633897 Free PMC article. Review.
Cited by
-
Modeling Skeletal Muscle Laminopathies Using Human Induced Pluripotent Stem Cells Carrying Pathogenic LMNA Mutations.Front Physiol. 2018 Oct 15;9:1332. doi: 10.3389/fphys.2018.01332. eCollection 2018. Front Physiol. 2018. PMID: 30405424 Free PMC article.
-
Involvement of muscle satellite cell dysfunction in neuromuscular disorders: Expanding the portfolio of satellite cell-opathies.Eur J Transl Myol. 2022 Mar 18;32(1):10064. doi: 10.4081/ejtm.2022.10064. Eur J Transl Myol. 2022. PMID: 35302338 Free PMC article.
-
Suppression of myopathic lamin mutations by muscle-specific activation of AMPK and modulation of downstream signaling.Hum Mol Genet. 2019 Feb 1;28(3):351-371. doi: 10.1093/hmg/ddy332. Hum Mol Genet. 2019. PMID: 30239736 Free PMC article.
-
Samp1 Mislocalization in Emery-Dreifuss Muscular Dystrophy.Cells. 2018 Oct 15;7(10):170. doi: 10.3390/cells7100170. Cells. 2018. PMID: 30326651 Free PMC article.
-
Impaired activity of the fusogenic micropeptide Myomixer causes myopathy resembling Carey-Fineman-Ziter syndrome.J Clin Invest. 2022 Jun 1;132(11):e159002. doi: 10.1172/JCI159002. J Clin Invest. 2022. PMID: 35642635 Free PMC article.
References
-
- Sewry CA. Muscular dystrophies: an update on pathology and diagnosis. Acta Neuropathol. 2010;120:343–358. - PubMed
-
- Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. - PubMed
-
- Bione S, Maestrini E, Rivella S, Mancini M, Regis S, et al. Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nat Genet. 1994;8:323–327. - PubMed
-
- Bonne G, Di Barletta MR, Varnous S, Becane HM, Hammouda EH, et al. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat Genet. 1999;21:285–288. - PubMed
-
- Muchir A, Bonne G, van der Kooi AJ, van Meegen M, Baas F, et al. Identification of mutations in the gene encoding lamins A/C in autosomal dominant limb girdle muscular dystrophy with atrioventricular conduction disturbances (LGMD1B). Hum Mol Genet. 2000;9:1453–1459. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

