CXCL12 inhibits expression of the NMDA receptor's NR2B subunit through a histone deacetylase-dependent pathway contributing to neuronal survival
- PMID: 21364640
- PMCID: PMC3032300
- DOI: 10.1038/cddis.2010.10
CXCL12 inhibits expression of the NMDA receptor's NR2B subunit through a histone deacetylase-dependent pathway contributing to neuronal survival
Abstract
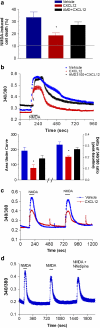
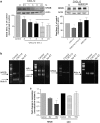
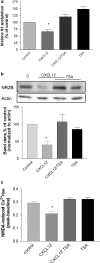
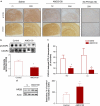
Homeostatic chemokines, such as CXCL12, can affect neuronal activity by the regulation of inhibitory and excitatory neurotransmission, but the mechanisms involved are still undefined. Our previous studies have shown that CXCL12 protects cortical neurons from excitotoxicity by promoting the function of the gene-repressor protein Rb, which is involved in the recruitment of chromatin modifiers (such as histone deacetylases (HDACs)) to gene promoters. In neurons, Rb controls activity-dependent genes essential to neuronal plasticity and survival, such as the N-methyl-D-aspartic acid (NMDA) receptor's subunit NR2B, the expression of which in the tetrameric ion channel largely affects calcium signaling by glutamate. In this study, we report that CXCL12 differentially modulates intracellular responses after stimulation of synaptic and extrasynaptic NMDA receptors, by a specific regulation of the NR2B gene that involves HDACs. Our results show that CXCL12 selectively inhibits NR2B expression in vitro and in vivo altering NMDA-induced calcium responses associated with neuronal death, while promoting prosurvival pathways that depend on stimulation of synaptic receptors. Along with previous studies, these findings underline the role of CXCL12/CXCR4 in the regulation of crucial components of glutamatergic transmission. These novel effects of CXCL12 may be involved in the physiological function of the chemokine in both developing and mature brains.
Figures

Similar articles
-
CXCL12-mediated regulation of ANP32A/Lanp, a component of the inhibitor of histone acetyl transferase (INHAT) complex, in cortical neurons.J Neuroimmune Pharmacol. 2011 Mar;6(1):163-70. doi: 10.1007/s11481-010-9228-5. Epub 2010 Jul 9. J Neuroimmune Pharmacol. 2011. PMID: 20617464 Free PMC article.
-
In developing hippocampal neurons, NR2B-containing N-methyl-D-aspartate receptors (NMDARs) can mediate signaling to neuronal survival and synaptic potentiation, as well as neuronal death.Neuroscience. 2009 Jan 12;158(1):334-43. doi: 10.1016/j.neuroscience.2008.01.080. Epub 2008 Mar 4. Neuroscience. 2009. PMID: 18378405 Free PMC article.
-
Involvement of the GluN2A and GluN2B subunits in synaptic and extrasynaptic N-methyl-D-aspartate receptor function and neuronal excitotoxicity.J Biol Chem. 2013 Aug 16;288(33):24151-9. doi: 10.1074/jbc.M113.482000. Epub 2013 Jul 9. J Biol Chem. 2013. PMID: 23839940 Free PMC article.
-
IGF-1-Involved Negative Feedback of NR2B NMDA Subunits Protects Cultured Hippocampal Neurons Against NMDA-Induced Excitotoxicity.Mol Neurobiol. 2017 Jan;54(1):684-696. doi: 10.1007/s12035-015-9647-7. Epub 2016 Jan 13. Mol Neurobiol. 2017. PMID: 26758454
-
The NR2B subtype of NMDA receptor: a potential target for the treatment of alcohol dependence.Curr Drug Targets CNS Neurol Disord. 2004 Jun;3(3):169-79. doi: 10.2174/1568007043337409. Curr Drug Targets CNS Neurol Disord. 2004. PMID: 15180478 Review.
Cited by
-
Bilaminar co-culture of primary rat cortical neurons and glia.J Vis Exp. 2011 Nov 12;(57):3257. doi: 10.3791/3257. J Vis Exp. 2011. PMID: 22105098 Free PMC article.
-
HIV-associated synaptic degeneration.Mol Brain. 2017 Aug 29;10(1):40. doi: 10.1186/s13041-017-0321-z. Mol Brain. 2017. PMID: 28851400 Free PMC article. Review.
-
Novel Positive Allosteric Modulators of Glutamate Transport Have Neuroprotective Properties in an in Vitro Excitotoxic Model.ACS Chem Neurosci. 2019 Aug 21;10(8):3437-3453. doi: 10.1021/acschemneuro.9b00061. Epub 2019 Jul 11. ACS Chem Neurosci. 2019. PMID: 31257852 Free PMC article.
-
CXCL12 chemokine and its receptors as major players in the interactions between immune and nervous systems.Front Cell Neurosci. 2014 Mar 6;8:65. doi: 10.3389/fncel.2014.00065. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24639628 Free PMC article. Review.
-
The effect of DPP-4 inhibition to improve functional outcome after stroke is mediated by the SDF-1α/CXCR4 pathway.Cardiovasc Diabetol. 2018 May 19;17(1):60. doi: 10.1186/s12933-018-0702-3. Cardiovasc Diabetol. 2018. PMID: 29776406 Free PMC article.
References
-
- Kaul M, Lipton SA. Mechanisms of neuronal injury and death in HIV-1 associated dementia. Curr HIV Res. 2006;4:307–318. - PubMed
-
- Greenwood SM, Connolly CN. Dendritic and mitochondrial changes during glutamate excitotoxicity. Neuropharmacology. 2007;53:891–898. - PubMed
-
- Waxman EA, Lynch DR. N-methyl-D-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005;11:37–49. - PubMed
-
- Wollmuth LP, Sobolevsky AI. Structure and gating of the glutamate receptor ion channel. Trends Neurosci. 2004;27:321–328. - PubMed
-
- Burnashev N. Calcium permeability of glutamate-gated channels in the central nervous system. Curr Opin Neurobiol. 1996;6:311–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

