Replication and recombination of herpes simplex virus DNA
- PMID: 21362621
- PMCID: PMC3091170
- DOI: 10.1074/jbc.R111.233981
Replication and recombination of herpes simplex virus DNA
Abstract
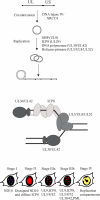
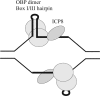
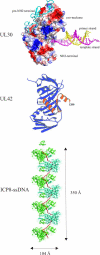
Replication of herpes simplex virus takes place in the cell nucleus and is carried out by a replisome composed of six viral proteins: the UL30-UL42 DNA polymerase, the UL5-UL8-UL52 helicase-primase, and the UL29 single-stranded DNA-binding protein ICP8. The replisome is loaded on origins of replication by the UL9 initiator origin-binding protein. Virus replication is intimately coupled to recombination and repair, often performed by cellular proteins. Here, we review new significant developments: the three-dimensional structures for the DNA polymerase, the polymerase accessory factor, and the single-stranded DNA-binding protein; the reconstitution of a functional replisome in vitro; the elucidation of the mechanism for activation of origins of DNA replication; the identification of cellular proteins actively involved in or responding to viral DNA replication; and the elucidation of requirements for formation of replication foci in the nucleus and effects on protein localization.
Figures
Similar articles
-
Herpes simplex viruses: mechanisms of DNA replication.Cold Spring Harb Perspect Biol. 2012 Sep 1;4(9):a013011. doi: 10.1101/cshperspect.a013011. Cold Spring Harb Perspect Biol. 2012. PMID: 22952399 Free PMC article. Review.
-
Functional order of assembly of herpes simplex virus DNA replication proteins into prereplicative site structures.J Virol. 1996 Mar;70(3):1759-67. doi: 10.1128/JVI.70.3.1759-1767.1996. J Virol. 1996. PMID: 8627698 Free PMC article.
-
HSV-1 DNA Replication-Coordinated Regulation by Viral and Cellular Factors.Viruses. 2021 Oct 7;13(10):2015. doi: 10.3390/v13102015. Viruses. 2021. PMID: 34696446 Free PMC article. Review.
-
Role of protein-protein interactions during herpes simplex virus type 1 recombination-dependent replication.J Biol Chem. 2004 May 21;279(21):21957-65. doi: 10.1074/jbc.M400832200. Epub 2004 Mar 16. J Biol Chem. 2004. PMID: 15026409
-
Herpes simplex virus 1 ICP8 mutant lacking annealing activity is deficient for viral DNA replication.Proc Natl Acad Sci U S A. 2019 Jan 15;116(3):1033-1042. doi: 10.1073/pnas.1817642116. Epub 2018 Dec 31. Proc Natl Acad Sci U S A. 2019. PMID: 30598436 Free PMC article.
Cited by
-
PNKP knockdown by RNA interference inhibits herpes simplex virus-1 replication in astrocytes.Virol Sin. 2013 Dec;28(6):345-51. doi: 10.1007/s12250-013-3350-5. Epub 2013 Nov 6. Virol Sin. 2013. PMID: 24213989 Free PMC article.
-
Viral gene drive in herpesviruses.Nat Commun. 2020 Sep 28;11(1):4884. doi: 10.1038/s41467-020-18678-0. Nat Commun. 2020. PMID: 32985507 Free PMC article.
-
Viral and cellular proteins containing FGDF motifs bind G3BP to block stress granule formation.PLoS Pathog. 2015 Feb 6;11(2):e1004659. doi: 10.1371/journal.ppat.1004659. eCollection 2015 Feb. PLoS Pathog. 2015. PMID: 25658430 Free PMC article.
-
Visualizing the replicating HSV-1 virus using STED super-resolution microscopy.Virol J. 2016 Apr 9;13:65. doi: 10.1186/s12985-016-0521-7. Virol J. 2016. PMID: 27062411 Free PMC article.
-
Mutational pressure by host APOBEC3s more strongly affects genes expressed early in the lytic phase of herpes simplex virus-1 (HSV-1) and human polyomavirus (HPyV) infection.PLoS Pathog. 2021 Apr 30;17(4):e1009560. doi: 10.1371/journal.ppat.1009560. eCollection 2021 Apr. PLoS Pathog. 2021. PMID: 33930088 Free PMC article.
References
-
- Lehman I. R., Boehmer P. E. (1999) J. Biol. Chem. 274, 28059–28062 - PubMed
-
- Shirata N., Kudoh A., Daikoku T., Tatsumi Y., Fujita M., Kiyono T., Sugaya Y., Isomura H., Ishizaki K., Tsurumi T. (2005) J. Biol. Chem. 280, 30336–30341 - PubMed
-
- Muylaert I., Elias P. (2007) J. Biol. Chem. 282, 10865–10872 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

