Hrs recognizes a hydrophobic amino acid cluster in cytokine receptors during ubiquitin-independent endosomal sorting
- PMID: 21362618
- PMCID: PMC3083181
- DOI: 10.1074/jbc.M110.191924
Hrs recognizes a hydrophobic amino acid cluster in cytokine receptors during ubiquitin-independent endosomal sorting
Abstract
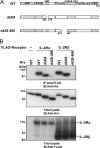
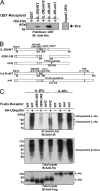
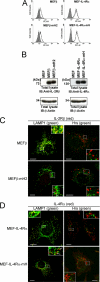
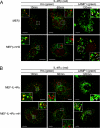
Hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs) is a component of the ESCRT-0 protein complex that captures ubiquitylated cargo proteins and sorts them to the lysosomal pathway. Although Hrs acts as a key transporter for ubiquitin-dependent endosomal sorting, we previously reported that Hrs is also involved in ubiquitin-independent endosomal sorting of interleukin-2 receptor β (IL-2Rβ). Here, we show direct interactions between bacterially expressed Hrs and interleukin-4 receptor α (IL-4Rα), indicating that their binding is not required for ubiquitylation of the receptors, similar to the case for IL-2Rβ. Examinations of the Hrs binding regions of the receptors reveal that a hydrophobic amino acid cluster in both IL-2Rβ and IL-4Rα is essential for the binding. Whereas the wild-type receptors are delivered to LAMP1-positive late endosomes, mutant receptors lacking the hydrophobic amino acid cluster are sorted to lysobisphosphatidic acid-positive late endosomes rather than LAMP1-positive late endosomes. We also show that the degradation of these mutant receptors is attenuated. Accordingly, Hrs functions during ubiquitin-independent endosomal sorting of the receptors by recognizing the hydrophobic amino acid cluster. These findings suggest the existence of a group of cargo proteins that have this hydrophobic amino acid cluster as a ubiquitin-independent sorting signal.
Figures


Similar articles
-
A hydrophobic amino acid cluster inserted into the C-terminus of a recycling cell surface receptor functions as an endosomal sorting signal.Biochem Biophys Res Commun. 2013 Nov 8;441(1):164-8. doi: 10.1016/j.bbrc.2013.10.019. Epub 2013 Oct 14. Biochem Biophys Res Commun. 2013. PMID: 24134837
-
Ubiquitin-independent binding of Hrs mediates endosomal sorting of the interleukin-2 receptor beta-chain.J Cell Sci. 2008 May 15;121(Pt 10):1727-38. doi: 10.1242/jcs.024455. Epub 2008 Apr 29. J Cell Sci. 2008. PMID: 18445679
-
ESCRT-0 protein hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs) is targeted to endosomes independently of signal-transducing adaptor molecule (STAM) and the complex formation with STAM promotes its endosomal dissociation.J Biol Chem. 2014 Nov 28;289(48):33296-310. doi: 10.1074/jbc.M114.578245. Epub 2014 Oct 8. J Biol Chem. 2014. PMID: 25296754 Free PMC article.
-
Hrs and endocytic sorting of ubiquitinated membrane proteins.Cell Struct Funct. 2002 Dec;27(6):403-8. doi: 10.1247/csf.27.403. Cell Struct Funct. 2002. PMID: 12576633 Review.
-
Endo-lysosomal sorting of G-protein-coupled receptors by ubiquitin: Diverse pathways for G-protein-coupled receptor destruction and beyond.Traffic. 2019 Feb;20(2):101-109. doi: 10.1111/tra.12619. Epub 2018 Nov 18. Traffic. 2019. PMID: 30353650 Free PMC article. Review.
Cited by
-
New Insights to Adenovirus-Directed Innate Immunity in Respiratory Epithelial Cells.Microorganisms. 2019 Jul 25;7(8):216. doi: 10.3390/microorganisms7080216. Microorganisms. 2019. PMID: 31349602 Free PMC article. Review.
-
The ESCRT-0 Protein HRS Interacts with the Human T Cell Leukemia Virus Type 2 Antisense Protein APH-2 and Suppresses Viral Replication.J Virol. 2019 Dec 12;94(1):e01311-19. doi: 10.1128/JVI.01311-19. Print 2019 Dec 12. J Virol. 2019. PMID: 31597781 Free PMC article.
-
Adenovirus early region 3 RIDα protein limits NFκB signaling through stress-activated EGF receptors.PLoS Pathog. 2019 Aug 19;15(8):e1008017. doi: 10.1371/journal.ppat.1008017. eCollection 2019 Aug. PLoS Pathog. 2019. PMID: 31425554 Free PMC article.
-
Commentary: IL-4 and IL-13 receptors and signaling.Cytokine. 2015 Sep;75(1):38-50. doi: 10.1016/j.cyto.2015.05.023. Epub 2015 Jul 14. Cytokine. 2015. PMID: 26187331 Free PMC article.
-
Molecular and cellular factors determining the functional pleiotropy of cytokines.FEBS J. 2023 May;290(10):2525-2552. doi: 10.1111/febs.16420. Epub 2022 Mar 14. FEBS J. 2023. PMID: 35246947 Free PMC article. Review.
References
-
- Bonifacino J. S., Traub L. M. (2003) Annu. Rev. Biochem. 72, 395–447 - PubMed
-
- Di Fiore P. P., Polo S., Hofmann K. (2003) Nat. Rev. Mol. Cell. Biol. 4, 491–497 - PubMed
-
- Hicke L., Schubert H. L., Hill C. P. (2005) Nat. Rev. Mol. Cell. Biol. 6, 610–621 - PubMed
-
- Katzmann D. J., Babst M., Emr S. D. (2001) Cell 106, 145–155 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

