Sprouty1 and Sprouty2 limit both the size of the otic placode and hindbrain Wnt8a by antagonizing FGF signaling
- PMID: 21362415
- PMCID: PMC3075364
- DOI: 10.1016/j.ydbio.2011.02.022
Sprouty1 and Sprouty2 limit both the size of the otic placode and hindbrain Wnt8a by antagonizing FGF signaling
Abstract
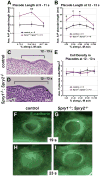
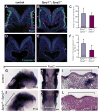
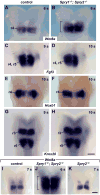
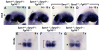
Multiple signaling molecules, including Fibroblast Growth Factor (FGF) and Wnt, induce two patches of ectoderm on either side of the hindbrain to form the progenitor cell population for the inner ear, or otic placode. Here we report that in Spry1, Spry2 compound mutant embryos (Spry1⁻/⁻; Spry2⁻/⁻ embryos), the otic placode is increased in size. We demonstrate that the otic placode is larger due to the recruitment of cells, normally destined to become cranial epidermis, into the otic domain. The enlargement of the otic placode observed in Spry1⁻/⁻; Spry2⁻/⁻ embryos is preceded by an expansion of a Wnt8a expression domain in the adjacent hindbrain. We demonstrate that both the enlargement of the otic placode and the expansion of the Wnt8a expression domain can be rescued in Spry1⁻/⁻; Spry2⁻/⁻ embryos by reducing the gene dosage of Fgf10. Our results define a FGF-responsive window during which cells can be continually recruited into the otic domain and uncover SPRY regulation of the size of a putative Wnt inductive center.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Cooperative and independent functions of FGF and Wnt signaling during early inner ear development.BMC Dev Biol. 2015 Oct 6;15:33. doi: 10.1186/s12861-015-0083-8. BMC Dev Biol. 2015. PMID: 26443994 Free PMC article.
-
Compensatory regulation of the size of the inner ear in response to excess induction of otic progenitors by fibroblast growth factor signaling.Dev Dyn. 2014 Oct;243(10):1317-27. doi: 10.1002/dvdy.24148. Epub 2014 Jun 12. Dev Dyn. 2014. PMID: 24847848 Free PMC article.
-
Roles of Wnt8a during formation and patterning of the mouse inner ear.Mech Dev. 2013 Feb;130(2-3):160-8. doi: 10.1016/j.mod.2012.09.009. Epub 2012 Oct 4. Mech Dev. 2013. PMID: 23041177
-
The first steps towards hearing: mechanisms of otic placode induction.Int J Dev Biol. 2007;51(6-7):463-72. doi: 10.1387/ijdb.072320to. Int J Dev Biol. 2007. PMID: 17891709 Review.
-
Changing shape and shaping change: Inducing the inner ear.Semin Cell Dev Biol. 2017 May;65:39-46. doi: 10.1016/j.semcdb.2016.10.006. Epub 2016 Oct 29. Semin Cell Dev Biol. 2017. PMID: 27989562 Review.
Cited by
-
The Fibroblast Growth Factor signaling pathway.Wiley Interdiscip Rev Dev Biol. 2015 May-Jun;4(3):215-66. doi: 10.1002/wdev.176. Epub 2015 Mar 13. Wiley Interdiscip Rev Dev Biol. 2015. PMID: 25772309 Free PMC article. Review.
-
Analysis of FGF-dependent and FGF-independent pathways in otic placode induction.PLoS One. 2013;8(1):e55011. doi: 10.1371/journal.pone.0055011. Epub 2013 Jan 23. PLoS One. 2013. PMID: 23355906 Free PMC article.
-
Making sense of Wnt signaling-linking hair cell regeneration to development.Front Cell Neurosci. 2015 Mar 11;9:66. doi: 10.3389/fncel.2015.00066. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25814927 Free PMC article. Review.
-
Effects of genetic variants of the bovine WNT8A gene on nine important growth traits in beef cattle.J Genet. 2017 Sep;96(4):535-544. doi: 10.1007/s12041-017-0804-9. J Genet. 2017. PMID: 28947701
-
Sprouty1 is a broad mediator of cellular senescence.Cell Death Dis. 2024 Apr 26;15(4):296. doi: 10.1038/s41419-024-06689-4. Cell Death Dis. 2024. PMID: 38670941 Free PMC article.
References
-
- Alvarez Y, Alonso MT, Vendrell V, Zelarayan LC, Chamero P, Theil T, Bosl MR, Kato S, Maconochie M, Riethmacher D, Schimmang T. Requirements for FGF3 and FGF10 during inner ear formation. Development. 2003;130:6329–38. - PubMed
-
- Basson MA, Akbulut S, Watson-Johnson J, Simon R, Carroll TJ, Shakya R, Gross I, Martin GR, Lufkin T, McMahon AP, Wilson PD, Costantini FD, Mason IJ, Licht JD. Sprouty1 Is a Critical Regulator of GDNF/RET-Mediated Kidney Induction. Dev Cell. 2005;8:229–239. - PubMed
-
- Brent AE, Tabin CJ. FGF acts directly on the somitic tendon progenitors through the Ets transcription factors Pea3 and Erm to regulate scleraxis expression. Development. 2004;131:3885–96. - PubMed
-
- Brown ST, Wang J, Groves AK. Dlx gene expression during chick inner ear development. J Comp Neurol. 2005;483:48–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

