Engineered cartilage maturation regulates cytokine production and interleukin-1β response
- PMID: 21359590
- PMCID: PMC3171533
- DOI: 10.1007/s11999-011-1826-x
Engineered cartilage maturation regulates cytokine production and interleukin-1β response
Abstract
Background: Because the injured joint has an actively inflammatory environment, the survival and repair potential of cartilage grafts may be influenced by inflammatory processes. Understanding the interactions of those processes with the graft may lead to concepts for pharmacologic or surgical solutions allowing improved cartilage repair.
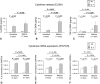
Questions/purposes: We asked whether the maturation level of cartilaginous tissues generated in vitro by expanded human articular chondrocytes (HACs) modulate (1) the spontaneous production of cytokines and (2) the response to interleukin (IL)-1β.
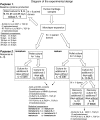
Methods: Twelve pellets/donor prepared with monolayer-expanded HACs (n = 6 donors) were evaluated at six different culture times for mRNA expression (n = 72) and spontaneous baseline release of monocyte chemoattractant protein (MCP)-1, IL-8, and transforming growth factor (TGF)-β1 (n = 72). We cultured 24 pellets/donor from each of four donors for 1 or 14 days (defined as immature and mature, respectively) and exposed the pellets to IL-1β for 3 days. MCP-1, IL-8, TGF-β1, and metalloprotease (MMP)-1 and MMP-13 were quantified in pellets and culture supernatants.
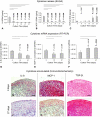
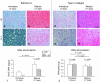
Results: By increasing culture time, the spontaneous release of IL-8 and MCP-1 decreased (12.0- and 5.5-fold, respectively), whereas that of TGF-β1 increased (5.4-fold). As compared with immature pellets, mature pellets responded to IL-1β by releasing lower amounts of MMP-1 (2.9-fold) and MMP-13 (1.7-fold) and increased levels of IL-8, MCP-1, and TGF-β1 (1.5-, 5.0-, and 7.5-fold, respectively). IL-8 and MCP-1 promptly returned to baseline on withdrawal of IL-1β.
Conclusions: Our observations suggest more mature cartilaginous tissues are more resistant to IL-1β exposure and can activate chemokines required to initiate tissue repair processes.
Clinical relevance: The implantation of more mature cartilaginous tissues might provide superior graft survival and improve/accelerate cartilage repair.
Figures
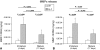


Similar articles
-
Physioxia Has a Beneficial Effect on Cartilage Matrix Production in Interleukin-1 Beta-Inhibited Mesenchymal Stem Cell Chondrogenesis.Cells. 2019 Aug 20;8(8):936. doi: 10.3390/cells8080936. Cells. 2019. PMID: 31434236 Free PMC article.
-
Pro-inflammatory IL-1beta and/or TNF-alpha up-regulate matrix metalloproteases-1 and -3 mRNA in chondrocyte subpopulations potentially pathogenic in osteoarthritis: in situ hybridization studies on a single cell level.Int J Rheum Dis. 2016 Jun;19(6):557-66. doi: 10.1111/1756-185X.12431. Epub 2014 Oct 8. Int J Rheum Dis. 2016. PMID: 25291965
-
Response of human engineered cartilage based on articular or nasal chondrocytes to interleukin-1β and low oxygen.Tissue Eng Part A. 2012 Feb;18(3-4):362-72. doi: 10.1089/ten.TEA.2011.0234. Epub 2011 Nov 4. Tissue Eng Part A. 2012. PMID: 21902467 Free PMC article.
-
Anabolic actions of IGF-I and TGF-beta1 on Interleukin-1beta-treated human articular chondrocytes: evaluation in two and three dimensional cultures.Histol Histopathol. 2009 Oct;24(10):1245-62. doi: 10.14670/HH-24.1245. Histol Histopathol. 2009. PMID: 19688693
-
Chemokine production by human chondrocytes.J Rheumatol. 1999 Sep;26(9):1992-2001. J Rheumatol. 1999. PMID: 10493682
Cited by
-
sb203580 preconditioning recharges matrix-expanded human adult stem cells for chondrogenesis in an inflammatory environment - A feasible approach for autologous stem cell based osteoarthritic cartilage repair.Biomaterials. 2015 Sep;64:88-97. doi: 10.1016/j.biomaterials.2015.06.038. Epub 2015 Jun 20. Biomaterials. 2015. PMID: 26122165 Free PMC article.
-
Muscle cell-derived factors inhibit inflammatory stimuli-induced damage in hMSC-derived chondrocytes.Osteoarthritis Cartilage. 2013 Jul;21(7):990-8. doi: 10.1016/j.joca.2013.04.011. Epub 2013 Apr 20. Osteoarthritis Cartilage. 2013. PMID: 23611899 Free PMC article.
-
Anti-inflammatory and pro-anabolic effects of 5-aminosalicylic acid on human inflammatory osteoarthritis models.J Orthop Translat. 2022 Oct 29;38:106-116. doi: 10.1016/j.jot.2022.10.003. eCollection 2023 Jan. J Orthop Translat. 2022. PMID: 36381242 Free PMC article.
-
Articular Cartilage Regeneration in Osteoarthritis.Cells. 2019 Oct 23;8(11):1305. doi: 10.3390/cells8111305. Cells. 2019. PMID: 31652798 Free PMC article. Review.
-
Chondrocyte and mesenchymal stem cell derived engineered cartilage exhibits differential sensitivity to pro-inflammatory cytokines.J Orthop Res. 2018 Nov;36(11):2901-2910. doi: 10.1002/jor.24061. Epub 2018 Jul 13. J Orthop Res. 2018. PMID: 29809295 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

