The influenza virus RNA synthesis machine: advances in its structure and function
- PMID: 21358279
- PMCID: PMC3127100
- DOI: 10.4161/rna.8.2.14513
The influenza virus RNA synthesis machine: advances in its structure and function
Abstract
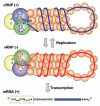
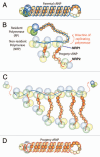
The influenza A viruses are the causative agents of respiratory disease that occurs as yearly epidemics and occasional pandemics. These viruses are endemic in wild avian species and can sometimes break the species barrier to infect and generate new virus lineages in humans. The influenza A virus genome consists of eight single-stranded, negative-polarity RNAs that form ribonucleoprotein complexes by association to the RNA polymerase and the nucleoprotein. In this review we focus on the structure of this RNA-synthesis machines and the included RNA polymerase, and on the mechanisms by which they express their genetic information as mRNAs and generate progeny ribonucleoproteins that will become incorporated into new infectious virions. New structural, biochemical and genetic data are rapidly accumulating in this very active area of research. We discuss these results and attempt to integrate the information into structural and functional models that may help the design of new experiments and further our knowledge on virus RNA replication and gene expression. This interplay between structural and functional data will eventually provide new targets for controlled attenuation or antiviral therapy.
Figures



Similar articles
-
Influenza virus transcription and replication.Adv Virus Res. 2013;87:113-37. doi: 10.1016/B978-0-12-407698-3.00004-1. Adv Virus Res. 2013. PMID: 23809922
-
Influenza virus RNA polymerase: insights into the mechanisms of viral RNA synthesis.Nat Rev Microbiol. 2016 Aug;14(8):479-93. doi: 10.1038/nrmicro.2016.87. Epub 2016 Jul 11. Nat Rev Microbiol. 2016. PMID: 27396566 Free PMC article. Review.
-
Structure and assembly of the influenza A virus ribonucleoprotein complex.FEBS Lett. 2013 Apr 17;587(8):1206-14. doi: 10.1016/j.febslet.2013.02.048. Epub 2013 Mar 13. FEBS Lett. 2013. PMID: 23499938 Review.
-
RNA-Free and Ribonucleoprotein-Associated Influenza Virus Polymerases Directly Bind the Serine-5-Phosphorylated Carboxyl-Terminal Domain of Host RNA Polymerase II.J Virol. 2016 Jun 10;90(13):6014-6021. doi: 10.1128/JVI.00494-16. Print 2016 Jul 1. J Virol. 2016. PMID: 27099314 Free PMC article.
-
The structure of a biologically active influenza virus ribonucleoprotein complex.PLoS Pathog. 2009 Jun;5(6):e1000491. doi: 10.1371/journal.ppat.1000491. Epub 2009 Jun 26. PLoS Pathog. 2009. PMID: 19557158 Free PMC article.
Cited by
-
Characterization of a Novel Orthomyxo-like Virus Causing Mass Die-Offs of Tilapia.mBio. 2016 Apr 5;7(2):e00431-16. doi: 10.1128/mBio.00431-16. mBio. 2016. PMID: 27048802 Free PMC article.
-
Biogenesis, assembly, and export of viral messenger ribonucleoproteins in the influenza A virus infected cell.RNA Biol. 2013 Aug;10(8):1274-82. doi: 10.4161/rna.25356. Epub 2013 Jun 17. RNA Biol. 2013. PMID: 23807439 Free PMC article. Review.
-
Modulation of Innate Immune Responses by the Influenza A NS1 and PA-X Proteins.Viruses. 2018 Dec 12;10(12):708. doi: 10.3390/v10120708. Viruses. 2018. PMID: 30545063 Free PMC article. Review.
-
Temperature Sensitive Mutations in Influenza A Viral Ribonucleoprotein Complex Responsible for the Attenuation of the Live Attenuated Influenza Vaccine.Viruses. 2018 Oct 15;10(10):560. doi: 10.3390/v10100560. Viruses. 2018. PMID: 30326610 Free PMC article. Review.
-
Host restriction of influenza virus polymerase activity by PB2 627E is diminished on short viral templates in a nucleoprotein-independent manner.J Virol. 2014 Jan;88(1):339-44. doi: 10.1128/JVI.02022-13. Epub 2013 Oct 23. J Virol. 2014. PMID: 24155385 Free PMC article.
References
-
- Horimoto T, Kawaoka Y. Influenza: lessons from past pandemics, warnings from current incidents. Nat Rev Microbiol. 2005;3:591–600. - PubMed
-
- Palese P, Shaw M. Orthomyxoviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. 5th edition. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 1647–1689.
-
- Baigent SJ, McCauley JW. Influenza type A in humans, mammals and birds: determinants of virus virulence, host-range and interspecies transmission. Bioessays. 2003;25:657–671. - PubMed
-
- Elton D, Digard P, Tiley L, Ortín J. Structure and function of the influenza virus RNP. In: Kawaoka Y, editor. Current Topics in Influenza Virology. Norfolk: Horizon Scientific Press; 2005. pp. 1–92.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
