Premature chromosome condensation induced by caffeine, 2-aminopurine, staurosporine and sodium metavanadate in S-phase arrested HeLa cells is associated with a decrease in Chk1 phosphorylation, formation of phospho-H2AX and minor cytoskeletal rearrangements
- PMID: 21347609
- PMCID: PMC3052479
- DOI: 10.1007/s00418-011-0793-3
Premature chromosome condensation induced by caffeine, 2-aminopurine, staurosporine and sodium metavanadate in S-phase arrested HeLa cells is associated with a decrease in Chk1 phosphorylation, formation of phospho-H2AX and minor cytoskeletal rearrangements
Abstract
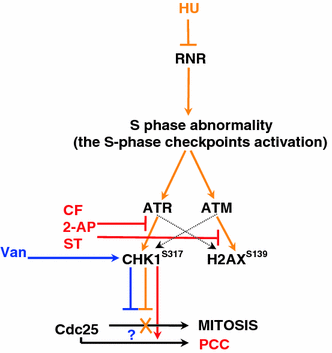
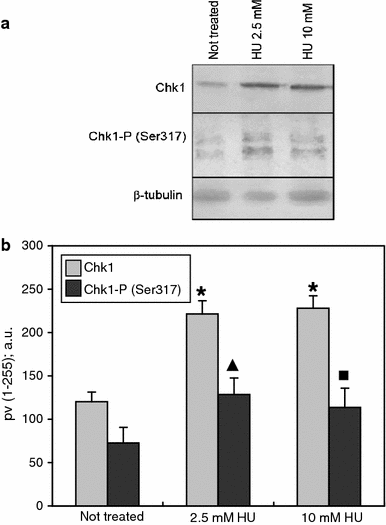
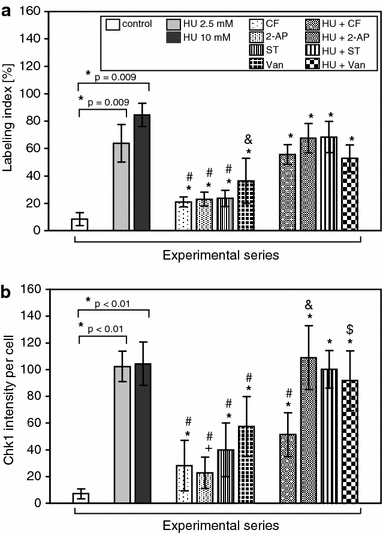
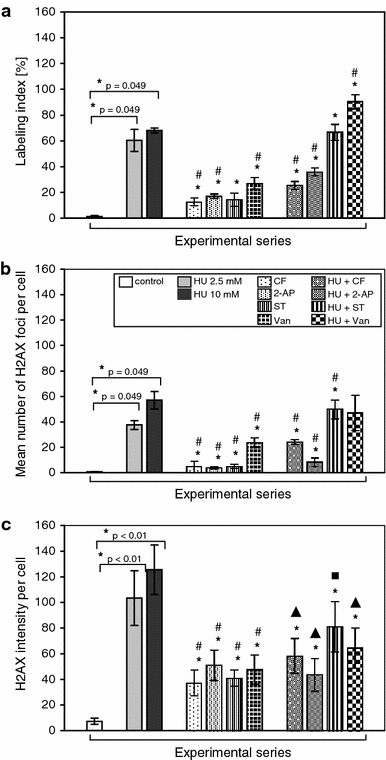
Here, we demonstrate that in HeLa cells, Ser317 of Chk1 undergoes phosphorylation in response to replication stress induced by hydroxyurea. We also demonstrate the existence of constitutive (interphase and mitotic) Chk1 kinase phosphorylation, the translocation of its phosphorylated form from the nucleus to cytoplasm in prometaphase as well as strong labeling of apoptotic nuclei with α-Chk1(S317) antibodies. Additionally, we show that caffeine, 2-aminopurine, staurosporine and sodium metavanadate can induce premature chromosome condensation (PCC) by the abrogation of the S-M checkpoint. Staurosporine appeared to be the most effective PCC inductor, and as in the case of the remaining inductors, the addition of hydroxyurea each time brought about an increase in the number of cells showing PCC symptoms (synergic effect). The forced premature mitosis was accompanied by an increasing index of double-strand breaks marked by the phosphorylation of histone H2AX on Ser139. Moreover, we found that the chemicals used brought about minor actin and tubulin network rearrangements that occurred following either replication stress or drug-induced cell cycle delay. At the same time, it was found that the extent of the cytoskeleton rearrangement did not hinder PCC in all its subperiods, i.e., from PCC-type prophase to PCC-type telophase.
Figures
Similar articles
-
DNA replication stress in CHK1-depleted tumour cells triggers premature (S-phase) mitosis through inappropriate activation of Aurora kinase B.Cell Death Dis. 2014 May 22;5(5):e1253. doi: 10.1038/cddis.2014.231. Cell Death Dis. 2014. PMID: 24853431 Free PMC article.
-
Carcinogen-induced S-phase arrest is Chk1 mediated and caffeine sensitive.Cell Growth Differ. 2002 Feb;13(2):77-86. Cell Growth Differ. 2002. PMID: 11864911
-
Phosphorylation of H2AX histones in response to double-strand breaks and induction of premature chromatin condensation in hydroxyurea-treated root meristem cells of Raphanus sativus, Vicia faba, and Allium porrum.Protoplasma. 2007;230(1-2):31-9. doi: 10.1007/s00709-006-0192-0. Epub 2006 Nov 21. Protoplasma. 2007. PMID: 17111099
-
Inhibition of human Chk1 causes increased initiation of DNA replication, phosphorylation of ATR targets, and DNA breakage.Mol Cell Biol. 2005 May;25(9):3553-62. doi: 10.1128/MCB.25.9.3553-3562.2005. Mol Cell Biol. 2005. PMID: 15831461 Free PMC article.
-
Chromosome condensation outside of mitosis: mechanisms and new tools.J Cell Physiol. 2006 Nov;209(2):297-304. doi: 10.1002/jcp.20720. J Cell Physiol. 2006. PMID: 16810672 Review.
Cited by
-
Genotoxicity assessment of two common curing weeds: Hyptis suaveolens (L.) Poir. and Leucas indica (L.) R. Br.Cytotechnology. 2016 Aug;68(4):1513-27. doi: 10.1007/s10616-015-9911-8. Epub 2015 Aug 19. Cytotechnology. 2016. PMID: 26286182 Free PMC article.
-
The Influence of PARP, ATR, CHK1 Inhibitors on Premature Mitotic Entry and Genomic Instability in High-Grade Serous BRCAMUT and BRCAWT Ovarian Cancer Cells.Cells. 2022 Jun 10;11(12):1889. doi: 10.3390/cells11121889. Cells. 2022. PMID: 35741017 Free PMC article.
-
Kinetics of DNA Repair in Vicia faba Meristem Regeneration Following Replication Stress.Cells. 2021 Jan 7;10(1):88. doi: 10.3390/cells10010088. Cells. 2021. PMID: 33430297 Free PMC article.
-
Ultrastructural changes associated with the induction of premature chromosome condensation in Vicia faba root meristem cells.Plant Cell Rep. 2014 Sep;33(9):1547-64. doi: 10.1007/s00299-014-1637-0. Epub 2014 Jun 5. Plant Cell Rep. 2014. PMID: 24898011 Free PMC article.
-
Participation of the ATR/CHK1 pathway in replicative stress targeted therapy of high-grade ovarian cancer.J Hematol Oncol. 2020 Apr 21;13(1):39. doi: 10.1186/s13045-020-00874-6. J Hematol Oncol. 2020. PMID: 32316968 Free PMC article. Review.
References
-
- Ajiro K, Yasuda H, Tsuji H. Vanadate triggers the transition from chromosome condensation to decondensation in a mitotic mutant (tsTM13) inactivation of p34cdc2/H1 kinase and dephosphorylation of mitosis-specific histone H3. Eur J Biochem. 1996;241:923–930. doi: 10.1111/j.1432-1033.1996.00923.x. - DOI - PubMed
-
- Andreassen PR, Margolis RL. Induction of partial mitosis in BHK cells by 2-aminopurine. J Cell Sci. 1991;100:290–310. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

