Cell origin of human mesenchymal stem cells determines a different healing performance in cardiac regeneration
- PMID: 21347366
- PMCID: PMC3037376
- DOI: 10.1371/journal.pone.0015652
Cell origin of human mesenchymal stem cells determines a different healing performance in cardiac regeneration
Abstract
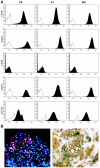
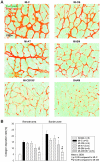
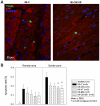
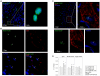
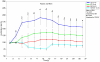
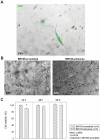
The possible different therapeutic efficacy of human mesenchymal stem cells (hMSC) derived from umbilical cord blood (CB), adipose tissue (AT) or bone marrow (BM) for the treatment of myocardial infarction (MI) remains unexplored. This study was to assess the regenerative potential of hMSC from different origins and to evaluate the role of CD105 in cardiac regeneration. Male SCID mice underwent LAD-ligation and received the respective cell type (400.000/per animal) intramyocardially. Six weeks post infarction, cardiac catheterization showed significant preservation of left ventricular functions in BM and CD105(+)-CB treated groups compared to CB and nontreated MI group (MI-C). Cell survival analyzed by quantitative real time PCR for human GAPDH and capillary density measured by immunostaining showed consistent results. Furthermore, cardiac remodeling can be significantly attenuated by BM-hMSC compared to MI-C. Under hypoxic conditions in vitro, remarkably increased extracellular acidification and apoptosis has been detected from CB-hMSC compared to BM and CD105 purified CB-derived hMSC. Our findings suggests that hMSC originating from different sources showed a different healing performance in cardiac regeneration and CD105(+) hMSC exhibited a favorable survival pattern in infarcted hearts, which translates into a more robust preservation of cardiac function.
Conflict of interest statement
Figures



Similar articles
-
Patterning human stem cells and endothelial cells with laser printing for cardiac regeneration.Biomaterials. 2011 Dec;32(35):9218-30. doi: 10.1016/j.biomaterials.2011.08.071. Epub 2011 Sep 10. Biomaterials. 2011. PMID: 21911255
-
Genetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium.Circ Res. 2010 Jun 11;106(11):1753-62. doi: 10.1161/CIRCRESAHA.109.196030. Epub 2010 Apr 8. Circ Res. 2010. PMID: 20378860 Free PMC article.
-
Chitosan/silk fibroin modified nanofibrous patches with mesenchymal stem cells prevent heart remodeling post-myocardial infarction in rats.Acta Biomater. 2018 Oct 15;80:154-168. doi: 10.1016/j.actbio.2018.09.013. Epub 2018 Sep 13. Acta Biomater. 2018. PMID: 30218777
-
Repair mechanisms of bone marrow mesenchymal stem cells in myocardial infarction.J Cell Mol Med. 2011 May;15(5):1032-43. doi: 10.1111/j.1582-4934.2010.01255.x. J Cell Mol Med. 2011. PMID: 21199333 Free PMC article. Review.
-
Mesenchymal stem cell therapy: A promising cell-based therapy for treatment of myocardial infarction.J Gene Med. 2017 Dec;19(12). doi: 10.1002/jgm.2995. Epub 2017 Dec 8. J Gene Med. 2017. PMID: 29044850 Review.
Cited by
-
The impact of cell source, culture methodology, culture location, and individual donors on gene expression profiles of bone marrow-derived and adipose-derived stromal cells.Stem Cells Dev. 2013 Apr 1;22(7):1086-96. doi: 10.1089/scd.2012.0384. Epub 2012 Dec 21. Stem Cells Dev. 2013. PMID: 23145933 Free PMC article.
-
Characterization of mononucleated human peripheral blood cells.ScientificWorldJournal. 2012;2012:843843. doi: 10.1100/2012/843843. Epub 2012 May 2. ScientificWorldJournal. 2012. PMID: 22666162 Free PMC article.
-
Distribution of cardiac stem cells in the human heart.ISRN Cardiol. 2012;2012:483407. doi: 10.5402/2012/483407. Epub 2012 Mar 4. ISRN Cardiol. 2012. PMID: 22462025 Free PMC article.
-
Differentiation Capacity of Human Urine-Derived Stem Cells to Retain Telomerase Activity.Front Cell Dev Biol. 2022 May 23;10:890574. doi: 10.3389/fcell.2022.890574. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35693947 Free PMC article.
-
Human Mesenchymal Stem Cells Display Reduced Expression of CD105 after Culture in Serum-Free Medium.Stem Cells Int. 2013;2013:698076. doi: 10.1155/2013/698076. Epub 2013 Sep 30. Stem Cells Int. 2013. PMID: 24194767 Free PMC article.
References
-
- Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, et al. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med. 1998;4:929–933. - PubMed
-
- Jia ZQ, Mickle DA, Weisel RD, Mohabeer MK, Merante F, et al. Transplanted cardiomyocytes survive in scar tissue and improve heart function. Transplant Proc. 1997;29:2093–2094. - PubMed
-
- Li RK, Jia ZQ, Weisel RD, Mickle DA, Zhang J, et al. Cardiomyocyte transplantation improves heart function. Ann Thorac Surg. 1996;62:654–660; discussion 660-651. - PubMed
-
- Li RK, Jia ZQ, Weisel RD, Merante F, Mickle DA. Smooth muscle cell transplantation into myocardial scar tissue improves heart function. Journal of Molecular and Cellular Cardiology. 1999;31:513–522. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

