Pro-inflammatory cytokine/chemokine production by reovirus treated melanoma cells is PKR/NF-κB mediated and supports innate and adaptive anti-tumour immune priming
- PMID: 21338484
- PMCID: PMC3052210
- DOI: 10.1186/1476-4598-10-20
Pro-inflammatory cytokine/chemokine production by reovirus treated melanoma cells is PKR/NF-κB mediated and supports innate and adaptive anti-tumour immune priming
Abstract
Background: As well as inducing direct oncolysis, reovirus treatment of melanoma is associated with activation of innate and adaptive anti-tumour immune responses.
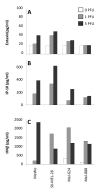
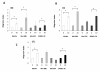
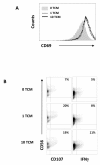
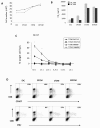
Results: Here we characterise the effects of conditioned media from reovirus-infected, dying human melanoma cells (reoTCM), in the absence of live virus, to address the immune bystander potential of reovirus therapy. In addition to RANTES, IL-8, MIP-1α and MIP-1β, reovirus-infected melanoma cells secreted eotaxin, IP-10 and the type 1 interferon IFN-β. To address the mechanisms responsible for the inflammatory composition of reoTCM, we show that IL-8 and IFN-β secretion by reovirus-infected melanoma cells was associated with activation of NF-κB and decreased by pre-treatment with small molecule inhibitors of NF-κB and PKR; specific siRNA-mediated knockdown further confirmed a role for PKR. This pro-inflammatory milieu induced a chemotactic response in isolated natural killer (NK) cells, dendritic cells (DC) and anti-melanoma cytotoxic T cells (CTL). Following culture in reoTCM, NK cells upregulated CD69 expression and acquired greater lytic potential against tumour targets. Furthermore, melanoma cell-loaded DC cultured in reoTCM were more effective at priming adaptive anti-tumour immunity.
Conclusions: These data demonstrate that the PKR- and NF-κB-dependent induction of pro-inflammatory molecules that accompanies reovirus-mediated killing can recruit and activate innate and adaptive effector cells, thus potentially altering the tumour microenvironment to support bystander immune-mediated therapy as well as direct viral oncolysis.
Figures


Similar articles
-
Reciprocal human dendritic cell-natural killer cell interactions induce antitumor activity following tumor cell infection by oncolytic reovirus.J Immunol. 2009 Oct 1;183(7):4312-21. doi: 10.4049/jimmunol.0901074. Epub 2009 Sep 4. J Immunol. 2009. PMID: 19734207
-
Reovirus activates human dendritic cells to promote innate antitumor immunity.J Immunol. 2008 May 1;180(9):6018-26. doi: 10.4049/jimmunol.180.9.6018. J Immunol. 2008. PMID: 18424722
-
Tumor infection by oncolytic reovirus primes adaptive antitumor immunity.Clin Cancer Res. 2008 Nov 15;14(22):7358-66. doi: 10.1158/1078-0432.CCR-08-0831. Clin Cancer Res. 2008. PMID: 19010851 Free PMC article.
-
Reovirus receptors, cell entry, and proapoptotic signaling.Adv Exp Med Biol. 2013;790:42-71. doi: 10.1007/978-1-4614-7651-1_3. Adv Exp Med Biol. 2013. PMID: 23884585 Free PMC article. Review.
-
Natural killer-dendritic cell cross-talk in cancer immunotherapy.Expert Opin Biol Ther. 2005 Oct;5(10):1303-15. doi: 10.1517/14712598.5.10.1303. Expert Opin Biol Ther. 2005. PMID: 16197336 Review.
Cited by
-
Diversion of stress granules and P-bodies during viral infection.Virology. 2013 Feb 20;436(2):255-67. doi: 10.1016/j.virol.2012.11.017. Epub 2013 Jan 3. Virology. 2013. PMID: 23290869 Free PMC article. Review.
-
Syngeneic Mouse Models for Pre-Clinical Evaluation of CAR T Cells.Cancers (Basel). 2024 Sep 18;16(18):3186. doi: 10.3390/cancers16183186. Cancers (Basel). 2024. PMID: 39335157 Free PMC article. Review.
-
Chemotherapy-Induced Senescence Reprogramming Promotes Nasopharyngeal Carcinoma Metastasis by circRNA-Mediated PKR Activation.Adv Sci (Weinh). 2023 Mar;10(8):e2205668. doi: 10.1002/advs.202205668. Epub 2023 Jan 22. Adv Sci (Weinh). 2023. PMID: 36683218 Free PMC article.
-
Changing faces in virology: the dutch shift from oncogenic to oncolytic viruses.Hum Gene Ther. 2014 Oct;25(10):875-84. doi: 10.1089/hum.2014.092. Epub 2014 Sep 17. Hum Gene Ther. 2014. PMID: 25141764 Free PMC article. Review.
-
Oncolytic Reovirus and Immune Checkpoint Inhibition as a Novel Immunotherapeutic Strategy for Breast Cancer.Cancers (Basel). 2018 Jun 15;10(6):205. doi: 10.3390/cancers10060205. Cancers (Basel). 2018. PMID: 29914097 Free PMC article.
References
-
- Harrington KJ, Karapanagiotou EM, Roulstone V, Twigger KR, White CL, Vidal L, Beirne D, Prestwich R, Newbold K, Ahmed M, Two-Stage Phase I Dose-Escalation Study of Intratumoral Reovirus Type 3 Dearing and Palliative Radiotherapy in Patients with Advanced Cancers. Clin Cancer Res. 2010. - PMC - PubMed
-
- Song L, Ohnuma T, Gelman IH, Holland JF. Reovirus infection of cancer cells is not due to activated Ras pathway. Cancer Gene Ther. 2008. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

