The traffic of proteins between nucleolar organizer regions and prenucleolar bodies governs the assembly of the nucleolus at exit of mitosis
- PMID: 21326952
- PMCID: PMC3030696
- DOI: 10.4161/nucl.1.2.11334
The traffic of proteins between nucleolar organizer regions and prenucleolar bodies governs the assembly of the nucleolus at exit of mitosis
Abstract
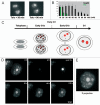
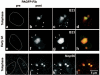
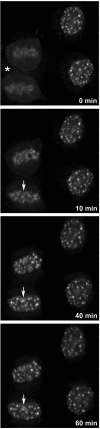
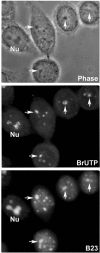
The building of nuclear bodies after mitosis is a coordinated event crucial for nuclear organization and function. The nucleolus is assembled during early G(1) phase. Here, two periods (early G1a and early G1b) have been defined. During these periods, the nucleolar compartments (DFC, GC) corresponding to different steps of ribosome biogenesis are progressively assembled. In telophase, rDNA transcription is first activated and PNBs (reservoirs of nucleolar processing proteins) are formed. The traffic of the processing proteins between incipient nucleoli and PNBs was analyzed using photoactivation. We demonstrate that the DFC protein fibrillarin passes from one incipient nucleolus to other nucleoli but not to PNBs, and that the GC proteins, B23/NPM and Nop52, shuttle between PNBs and incipient nucleoli. This difference in traffic suggests a way of regulating assembly first of DFC and then of GC. The time of residency of GC proteins is high in incipient nucleoli compared to interphase nuclei, it decreases in LMB-treated early G1a cells impairing the assembly of GC. Because the assembly of the nucleolus and that of the Cajal body at the exit from mitosis are both sensitive to CRM1 activity, we discuss the fact that assembly of GC and/or its interaction with DFC in early G1a depends on shuttling between PNBs and NORs in a manner dependent on Cajal body assembly.
Keywords: CRM1; Cajal body; EM structure; PNBs; cell cycle; living cell; nucleolus; photoactivation; traffic.
Figures

Similar articles
-
Nucleolar assembly of the rRNA processing machinery in living cells.J Cell Biol. 2001 May 28;153(5):1097-110. doi: 10.1083/jcb.153.5.1097. J Cell Biol. 2001. PMID: 11381093 Free PMC article.
-
Effects of anti-fibrillarin antibodies on building of functional nucleoli at the end of mitosis.J Cell Sci. 1998 Feb;111 ( Pt 3):359-72. doi: 10.1242/jcs.111.3.359. J Cell Sci. 1998. PMID: 9427684
-
Raptor, a positive regulatory subunit of mTOR complex 1, is a novel phosphoprotein of the rDNA transcription machinery in nucleoli and chromosomal nucleolus organizer regions (NORs).Cell Cycle. 2011 Sep 15;10(18):3140-52. doi: 10.4161/cc.10.18.17376. Epub 2011 Sep 15. Cell Cycle. 2011. PMID: 21900751
-
Assembly and disassembly of the nucleolus during the cell cycle.Nucleus. 2011 May-Jun;2(3):189-94. doi: 10.4161/nucl.2.3.16246. Nucleus. 2011. PMID: 21818412 Free PMC article. Review.
-
The functional organization of the nucleolus in proliferating plant cells.Eur J Histochem. 2000;44(2):117-31. Eur J Histochem. 2000. PMID: 10968360 Review.
Cited by
-
Determinants of mammalian nucleolar architecture.Chromosoma. 2015 Sep;124(3):323-31. doi: 10.1007/s00412-015-0507-z. Epub 2015 Feb 12. Chromosoma. 2015. PMID: 25670395 Free PMC article. Review.
-
Human nucleolar protein Nop52 (RRP1/NNP-1) is involved in site 2 cleavage in internal transcribed spacer 1 of pre-rRNAs at early stages of ribosome biogenesis.Nucleic Acids Res. 2015 Jun 23;43(11):5524-36. doi: 10.1093/nar/gkv470. Epub 2015 May 12. Nucleic Acids Res. 2015. PMID: 25969445 Free PMC article.
-
Nucleolar Division in the Promastigote Stage of Leishmania major Parasite: A Nop56 Point of View.Biomed Res Int. 2018 Oct 10;2018:1641839. doi: 10.1155/2018/1641839. eCollection 2018. Biomed Res Int. 2018. PMID: 30406129 Free PMC article.
-
Keeping up with the condensates: The retention, gain, and loss of nuclear membrane-less organelles.Front Mol Biosci. 2022 Sep 20;9:998363. doi: 10.3389/fmolb.2022.998363. eCollection 2022. Front Mol Biosci. 2022. PMID: 36203874 Free PMC article. Review.
-
ADRB3 induces mobilization and inhibits differentiation of both breast cancer cells and myeloid-derived suppressor cells.Cell Death Dis. 2022 Feb 10;13(2):141. doi: 10.1038/s41419-022-04603-4. Cell Death Dis. 2022. PMID: 35145073 Free PMC article.
References
-
- Spector DL. Nuclear domains. J Cell Sci. 2001;114:2891–2893. - PubMed
-
- Hernandez-Verdun D. The nucleolus: a model for the organization of nuclear functions. Histochem Cell Biol. 2006;126:135–148. - PubMed
-
- Gébrane-Younís J, Sirri V, Junéra HR, Roussel P, Hernandez-Verdun D. Diekmann. Visions of the cell nucleus: ASP CA; 2005. Nucleolus: An essential nuclear domain; pp. 120–135. PHaS, ed.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
