2-hydroxyglutarate production, but not dominant negative function, is conferred by glioma-derived NADP-dependent isocitrate dehydrogenase mutations
- PMID: 21326614
- PMCID: PMC3033901
- DOI: 10.1371/journal.pone.0016812
2-hydroxyglutarate production, but not dominant negative function, is conferred by glioma-derived NADP-dependent isocitrate dehydrogenase mutations
Abstract
Background: Gliomas frequently contain mutations in the cytoplasmic NADP(+)-dependent isocitrate dehydrogenase (IDH1) or the mitochondrial NADP(+)-dependent isocitrate dehydrogenase (IDH2). Several different amino acid substitutions recur at either IDH1 R132 or IDH2 R172 in glioma patients. Genetic evidence indicates that these mutations share a common gain of function, but it is unclear whether the shared function is dominant negative activity, neomorphic production of (R)-2-hydroxyglutarate (2HG), or both.
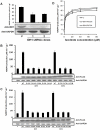
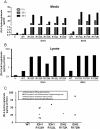
Methodology/principal findings: We show by coprecipitation that five cancer-derived IDH1 R132 mutants bind IDH1-WT but that three cancer-derived IDH2 R172 mutants exert minimal binding to IDH2-WT. None of the mutants dominant-negatively lower isocitrate dehydrogenase activity at physiological (40 µM) isocitrate concentrations in mammalian cell lysates. In contrast to this, all of these mutants confer 10- to 100-fold higher 2HG production to cells, and glioma tissues containing IDH1 R132 or IDH2 R172 mutations contain high levels of 2HG compared to glioma tissues without IDH mutations (54.4 vs. 0.1 mg 2HG/g protein).
Conclusions: Binding to, or dominant inhibition of, WT IDH1 or IDH2 is not a shared feature of the IDH1 and IDH2 mutations, and thus is not likely to be important in cancer. The fact that the gain of the enzymatic activity to produce 2HG is a shared feature of the IDH1 and IDH2 mutations suggests that this is an important function for these mutants in driving cancer pathogenesis.
Conflict of interest statement
Figures

Similar articles
-
Identification of additional IDH mutations associated with oncometabolite R(-)-2-hydroxyglutarate production.Oncogene. 2012 May 10;31(19):2491-8. doi: 10.1038/onc.2011.416. Epub 2011 Sep 26. Oncogene. 2012. PMID: 21996744 Free PMC article.
-
The potential for isocitrate dehydrogenase mutations to produce 2-hydroxyglutarate depends on allele specificity and subcellular compartmentalization.J Biol Chem. 2013 Feb 8;288(6):3804-15. doi: 10.1074/jbc.M112.435495. Epub 2012 Dec 21. J Biol Chem. 2013. PMID: 23264629 Free PMC article.
-
Isocitrate dehydrogenase mutation as a therapeutic target in gliomas.Chin Clin Oncol. 2017 Jun;6(3):33. doi: 10.21037/cco.2017.06.11. Chin Clin Oncol. 2017. PMID: 28705010
-
IDH mutations in glioma and acute myeloid leukemia.Trends Mol Med. 2010 Sep;16(9):387-97. doi: 10.1016/j.molmed.2010.07.002. Epub 2010 Aug 5. Trends Mol Med. 2010. PMID: 20692206 Review.
-
Isocitrate dehydrogenase mutations in gliomas.Neuro Oncol. 2016 Jan;18(1):16-26. doi: 10.1093/neuonc/nov136. Epub 2015 Jul 16. Neuro Oncol. 2016. PMID: 26188014 Free PMC article. Review.
Cited by
-
IDH mutations in human glioma.Neurosurg Clin N Am. 2012 Jul;23(3):471-80. doi: 10.1016/j.nec.2012.04.009. Epub 2012 May 31. Neurosurg Clin N Am. 2012. PMID: 22748659 Free PMC article. Review.
-
IDH1 p.R132 mutations may not be actively involved in the carcinogenesis of hepatocellular carcinoma.Med Sci Monit. 2014 Feb 14;20:247-54. doi: 10.12659/MSM.889891. Med Sci Monit. 2014. PMID: 24531386 Free PMC article.
-
A Noninvasive Comparison Study between Human Gliomas with IDH1 and IDH2 Mutations by MR Spectroscopy.Metabolites. 2019 Feb 20;9(2):35. doi: 10.3390/metabo9020035. Metabolites. 2019. PMID: 30791611 Free PMC article.
-
What do we know about IDH1/2 mutations so far, and how do we use it?Acta Neuropathol. 2013 May;125(5):621-36. doi: 10.1007/s00401-013-1106-9. Epub 2013 Mar 20. Acta Neuropathol. 2013. PMID: 23512379 Free PMC article. Review.
-
Design, mechanism of action, bioavailability and therapeutic effects of mn porphyrin-based redox modulators.Med Princ Pract. 2013;22(2):103-30. doi: 10.1159/000341715. Epub 2012 Oct 16. Med Princ Pract. 2013. PMID: 23075911 Free PMC article. Review.
References
-
- Green A, Beer P. Somatic mutations of IDH1 and IDH2 in the leukemic transformation of myeloproliferative neoplasms. N Engl J Med. 2010;362:369–370. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

