Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets
- PMID: 21317389
- PMCID: PMC3198034
- DOI: 10.4049/jimmunol.1003613
Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets
Abstract
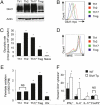
Stimulated CD4(+) T lymphocytes can differentiate into effector T cell (Teff) or inducible regulatory T cell (Treg) subsets with specific immunological roles. We show that Teff and Treg require distinct metabolic programs to support these functions. Th1, Th2, and Th17 cells expressed high surface levels of the glucose transporter Glut1 and were highly glycolytic. Treg, in contrast, expressed low levels of Glut1 and had high lipid oxidation rates. Consistent with glycolysis and lipid oxidation promoting Teff and Treg, respectively, Teff were selectively increased in Glut1 transgenic mice and reliant on glucose metabolism, whereas Treg had activated AMP-activated protein kinase and were dependent on lipid oxidation. Importantly, AMP-activated protein kinase stimulation was sufficient to decrease Glut1 and increase Treg generation in an asthma model. These data demonstrate that CD4(+) T cell subsets require distinct metabolic programs that can be manipulated in vivo to control Treg and Teff development in inflammatory diseases.
Figures
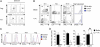
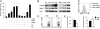
Similar articles
-
The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function.Cell Metab. 2014 Jul 1;20(1):61-72. doi: 10.1016/j.cmet.2014.05.004. Epub 2014 Jun 12. Cell Metab. 2014. PMID: 24930970 Free PMC article.
-
Foxp3 and Toll-like receptor signaling balance Treg cell anabolic metabolism for suppression.Nat Immunol. 2016 Dec;17(12):1459-1466. doi: 10.1038/ni.3577. Epub 2016 Oct 3. Nat Immunol. 2016. PMID: 27695003 Free PMC article.
-
In Vitro Differentiation of Effector CD4+ T Helper Cell Subsets.Methods Mol Biol. 2019;1960:75-84. doi: 10.1007/978-1-4939-9167-9_6. Methods Mol Biol. 2019. PMID: 30798522
-
mTOR signaling in the differentiation and function of regulatory and effector T cells.Curr Opin Immunol. 2017 Jun;46:103-111. doi: 10.1016/j.coi.2017.04.005. Epub 2017 May 20. Curr Opin Immunol. 2017. PMID: 28535458 Free PMC article. Review.
-
Metabolic control of the Treg/Th17 axis.Immunol Rev. 2013 Mar;252(1):52-77. doi: 10.1111/imr.12029. Immunol Rev. 2013. PMID: 23405895 Free PMC article. Review.
Cited by
-
Targeting immune cell metabolism in kidney diseases.Nat Rev Nephrol. 2021 Jul;17(7):465-480. doi: 10.1038/s41581-021-00413-7. Epub 2021 Apr 7. Nat Rev Nephrol. 2021. PMID: 33828286 Review.
-
Thymopoiesis, Alterations in Dendritic Cells and Tregs, and Reduced T Cell Activation in Successful Extracorporeal Photopheresis Treatment of GVHD.J Clin Immunol. 2021 Jul;41(5):1016-1030. doi: 10.1007/s10875-021-00991-y. Epub 2021 Mar 2. J Clin Immunol. 2021. PMID: 33651234 Free PMC article.
-
Immune metabolism in PD-1 blockade-based cancer immunotherapy.Int Immunol. 2021 Jan 1;33(1):17-26. doi: 10.1093/intimm/dxaa046. Int Immunol. 2021. PMID: 32622347 Free PMC article. Review.
-
Peptidoglycan recognition protein 1 enhances experimental asthma by promoting Th2 and Th17 and limiting regulatory T cell and plasmacytoid dendritic cell responses.J Immunol. 2013 Apr 1;190(7):3480-92. doi: 10.4049/jimmunol.1202675. Epub 2013 Feb 18. J Immunol. 2013. PMID: 23420883 Free PMC article.
-
Mitochondrial Inhibition: a Treatment Strategy in Cancer?Curr Oncol Rep. 2021 Mar 17;23(4):49. doi: 10.1007/s11912-021-01033-x. Curr Oncol Rep. 2021. PMID: 33730242 Review.
References
-
- Kronenberg M, Rudensky A. Regulation of immunity by self-reactive T cells. Nature. 2005;435:598–604. - PubMed
-
- Cham CM, Gajewski TF. Glucose availability regulates IFN-gamma production and p70S6 kinase activation in CD8+ effector T cells. J. Immunol. 2005;174:4670–4677. - PubMed
-
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

