HPRT deficiency coordinately dysregulates canonical Wnt and presenilin-1 signaling: a neuro-developmental regulatory role for a housekeeping gene?
- PMID: 21305049
- PMCID: PMC3030599
- DOI: 10.1371/journal.pone.0016572
HPRT deficiency coordinately dysregulates canonical Wnt and presenilin-1 signaling: a neuro-developmental regulatory role for a housekeeping gene?
Erratum in
- PLoS One. 2011;6(3). doi: 10.1371/annotation/893f09fd-ab24-4119-93f4-44a03a3342f9. Jinnah, H A [added]
Abstract
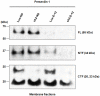
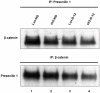
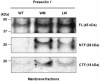
We have used microarray-based methods of global gene expression together with quantitative PCR and Western blot analysis to identify dysregulation of genes and aberrant cellular processes in human fibroblasts and in SH-SY5Y neuroblastoma cells made HPRT-deficient by transduction with a retrovirus stably expressing an shRNA targeted against HPRT. Analysis of the microarray expression data by Gene ontology (GO) and Gene Set Enrichment Analysis (GSEA) as well as significant pathway analysis by GeneSpring GX10 and Panther Classification System reveal that HPRT deficiency is accompanied by aberrations in a variety of pathways known to regulate neurogenesis or to be implicated in neurodegenerative disease, including the canonical Wnt/β-catenin and the Alzheimer's disease/presenilin signaling pathways. Dysregulation of the Wnt/β-catenin pathway is confirmed by Western blot demonstration of cytosolic sequestration of β-catenin during in vitro differentiation of the SH-SY5Y cells toward the neuronal phenotype. We also demonstrate that two key transcription factor genes known to be regulated by Wnt signaling and to be vital for the generation and function of dopaminergic neurons; i.e., Lmx1a and Engrailed 1, are down-regulated in the HPRT knockdown SH-SY5Y cells. In addition to the Wnt signaling aberration, we found that expression of presenilin-1 shows severely aberrant expression in HPRT-deficient SH-SY5Y cells, reflected by marked deficiency of the 23 kDa C-terminal fragment of presenilin-1 in knockdown cells. Western blot analysis of primary fibroblast cultures from two LND patients also shows dysregulated presenilin-1 expression, including aberrant proteolytic processing of presenilin-1. These demonstrations of dysregulated Wnt signaling and presenilin-1 expression together with impaired expression of dopaminergic transcription factors reveal broad pleitropic neuro-regulatory defects played by HPRT expression and suggest new directions for investigating mechanisms of aberrant neurogenesis and neuropathology in LND and potential new targets for restoration of effective signaling in this neuro-developmental defect.
Conflict of interest statement
Figures

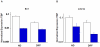
Similar articles
-
MicroRNA-mediated dysregulation of neural developmental genes in HPRT deficiency: clues for Lesch-Nyhan disease?Hum Mol Genet. 2012 Feb 1;21(3):609-22. doi: 10.1093/hmg/ddr495. Epub 2011 Oct 31. Hum Mol Genet. 2012. PMID: 22042773 Free PMC article.
-
Hypoxanthine-guanine phosphoribosyl transferase regulates early developmental programming of dopamine neurons: implications for Lesch-Nyhan disease pathogenesis.Hum Mol Genet. 2009 Jul 1;18(13):2317-27. doi: 10.1093/hmg/ddp164. Epub 2009 Apr 2. Hum Mol Genet. 2009. PMID: 19342420 Free PMC article.
-
Deficiency of the housekeeping gene hypoxanthine-guanine phosphoribosyltransferase (HPRT) dysregulates neurogenesis.Mol Ther. 2010 Jan;18(1):54-62. doi: 10.1038/mt.2009.178. Epub 2009 Aug 11. Mol Ther. 2010. PMID: 19672249 Free PMC article.
-
Wnt/β-Catenin Signaling Pathway Governs a Full Program for Dopaminergic Neuron Survival, Neurorescue and Regeneration in the MPTP Mouse Model of Parkinson's Disease.Int J Mol Sci. 2018 Nov 24;19(12):3743. doi: 10.3390/ijms19123743. Int J Mol Sci. 2018. PMID: 30477246 Free PMC article. Review.
-
[Deficiencies of hypoxanthine guanine phosphoribosyltransferase (HPRT)].Nihon Rinsho. 2008 Apr;66(4):687-93. Nihon Rinsho. 2008. PMID: 18409516 Review. Japanese.
Cited by
-
Purinergic signaling in human pluripotent stem cells is regulated by the housekeeping gene encoding hypoxanthine guanine phosphoribosyltransferase.Proc Natl Acad Sci U S A. 2012 Feb 28;109(9):3377-82. doi: 10.1073/pnas.1118067109. Epub 2012 Feb 13. Proc Natl Acad Sci U S A. 2012. PMID: 22331909 Free PMC article.
-
Toward a theory of childhood learning disorders, hyperactivity, and aggression.ISRN Psychiatry. 2012 Sep 27;2012:589792. doi: 10.5402/2012/589792. Print 2012. ISRN Psychiatry. 2012. PMID: 23762766 Free PMC article.
-
HPRT-deficiency dysregulates cAMP-PKA signaling and phosphodiesterase 10A expression: mechanistic insight and potential target for Lesch-Nyhan Disease?PLoS One. 2013 May 14;8(5):e63333. doi: 10.1371/journal.pone.0063333. Print 2013. PLoS One. 2013. PMID: 23691025 Free PMC article.
-
Cytosolic 5'-nucleotidase II interacts with the leucin rich repeat of NLR family member Ipaf.PLoS One. 2015 Mar 26;10(3):e0121525. doi: 10.1371/journal.pone.0121525. eCollection 2015. PLoS One. 2015. PMID: 25811392 Free PMC article.
-
MicroRNA-mediated dysregulation of neural developmental genes in HPRT deficiency: clues for Lesch-Nyhan disease?Hum Mol Genet. 2012 Feb 1;21(3):609-22. doi: 10.1093/hmg/ddr495. Epub 2011 Oct 31. Hum Mol Genet. 2012. PMID: 22042773 Free PMC article.
References
-
- Jinnah HA, Friedmann T. Lesch-Nyhan disease and its variants. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. 8 ed. New York: McGraw-Hill; 2000. pp. 2537–2570.
-
- Lloyd KG, Hornykiewicz O, Davidson L, Shannak K, Farley I, et al. Biochemical evidence of dysfunction of brain neurotransmitters in the Lesch-Nyhan syndrome. N Engl J Med. 1981;305:1106–1111. - PubMed
-
- Smith D, Friedmann T. Characterization of the dopamine defect in primary cultures of dopaminergic neurons from hypoxanthine phosphoribosyltransferase knockout mice. Mol Ther. 2000;1:486–491. - PubMed
-
- Boer P, Brosh S, Wasserman L, Hammel I, Zoref-Shani E, et al. Decelerated rate of dendrite outgrowth from dopaminergic neurons in primary cultures from brains of hypoxanthine phosphoribosyltransferase-deficient knockout mice. Neurosci Lett. 2001;303:45–48. - PubMed
-
- Ernst M, Zametkin AJ, Matochik JA, Pascualvaca D, Jons PH, et al. Presynaptic dopaminergic deficits in Lesch-Nyhan disease. N Engl J Med. 1996;334:1568–1572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

