Response to 2009 pandemic influenza A (H1N1) vaccine in HIV-infected patients and the influence of prior seasonal influenza vaccination
- PMID: 21304982
- PMCID: PMC3031580
- DOI: 10.1371/journal.pone.0016496
Response to 2009 pandemic influenza A (H1N1) vaccine in HIV-infected patients and the influence of prior seasonal influenza vaccination
Abstract
Background: The immunogenicity of 2009 pandemic influenza A(H1N1) (pH1N1) vaccines and the effect of previous influenza vaccination is a matter of current interest and debate. We measured the immune response to pH1N1 vaccine in HIV-infected patients and in healthy controls. In addition we tested whether recent vaccination with seasonal trivalent inactivated vaccine (TIV) induced cross-reactive antibodies to pH1N1. (clinicaltrials.gov Identifier:NCT01066169).
Methods and findings: In this single-center prospective cohort study MF59-adjuvanted pH1N1 vaccine (Focetria®, Novartis) was administered twice to 58 adult HIV-infected patients and 44 healthy controls in November 2009 (day 0 and day 21). Antibody responses were measured at baseline, day 21 and day 56 with hemagglutination-inhibition (HI) assay. The seroprotection rate (defined as HI titers ≥ 1 : 40) for HIV-infected patients was 88% after the first and 91% after the second vaccination. These rates were comparable to those in healthy controls. Post-vaccination GMT, a sensitive marker of the immune competence of a group, was lower in HIV-infected patients. We found a high seroprotection rate at baseline (31%). Seroprotective titers at baseline were much more common in those who had received 2009-2010 seasonal TIV three weeks prior to the first dose of pH1N1 vaccine. Using stored serum samples of 51 HIV-infected participants we measured the pH1N1 specific response to 2009-2010 seasonal TIV. The seroprotection rate to pH1N1 increased from 22% to 49% after vaccination with 2009-2010 seasonal TIV. Seasonal TIV induced higher levels of antibodies to pH1N1 in older than in younger subjects.
Conclusion: In HIV-infected patients on combination antiretroviral therapy, with a median CD4+ T-lymphocyte count above 500 cells/mm(3), one dose of MF59-adjuvanted pH1N1 vaccine induced a high seroprotection rate comparable to that in healthy controls. A second dose had a modest additional effect. Furthermore, seasonal TIV induced cross-reactive antibodies to pH1N1 and this effect was more pronounced in older subjects.
Conflict of interest statement
Figures
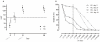
Similar articles
-
Long-term immunogenicity after one and two doses of a monovalent MF59-adjuvanted A/H1N1 Influenza virus vaccine coadministered with the seasonal 2009-2010 nonadjuvanted Influenza virus vaccine in HIV-infected children, adolescents, and young adults in a randomized controlled trial.Clin Vaccine Immunol. 2011 Sep;18(9):1503-9. doi: 10.1128/CVI.05200-11. Epub 2011 Jul 27. Clin Vaccine Immunol. 2011. PMID: 21795458 Free PMC article. Clinical Trial.
-
Response to 2009 pandemic and seasonal influenza vaccines co-administered to HIV-infected and HIV-uninfected former drug users living in a rehabilitation community in Italy.Vaccine. 2011 Nov 15;29(49):9209-13. doi: 10.1016/j.vaccine.2011.09.103. Epub 2011 Oct 3. Vaccine. 2011. PMID: 21974995
-
Responses to pandemic ASO3-adjuvanted A/California/07/09 H1N1 influenza vaccine in human immunodeficiency virus-infected individuals.BMC Immunol. 2012 Aug 31;13:49. doi: 10.1186/1471-2172-13-49. BMC Immunol. 2012. PMID: 22937824 Free PMC article.
-
Immunogenicity and safety of pandemic influenza A (H1N1) 2009 vaccine: systematic review and meta-analysis.Influenza Other Respir Viruses. 2011 Sep;5(5):299-305. doi: 10.1111/j.1750-2659.2011.00229.x. Epub 2011 Mar 21. Influenza Other Respir Viruses. 2011. PMID: 21668694 Free PMC article. Review.
-
Influenza and HIV: lessons from the 2009 H1N1 influenza pandemic.Curr HIV/AIDS Rep. 2011 Sep;8(3):181-91. doi: 10.1007/s11904-011-0086-4. Curr HIV/AIDS Rep. 2011. PMID: 21710214 Review.
Cited by
-
Serological response of patients with influenza A (H1N1) pdm09-associated pneumonia: an observational study.PLoS One. 2013 Nov 27;8(11):e81436. doi: 10.1371/journal.pone.0081436. eCollection 2013. PLoS One. 2013. PMID: 24312299 Free PMC article.
-
Vaccination of HIV-infected pregnant women: implications for protection of their young infants.Trop Dis Travel Med Vaccines. 2017 Jan 6;3:1. doi: 10.1186/s40794-016-0044-7. eCollection 2017. Trop Dis Travel Med Vaccines. 2017. PMID: 28883971 Free PMC article. Review.
-
Long-term immunogenicity after one and two doses of a monovalent MF59-adjuvanted A/H1N1 Influenza virus vaccine coadministered with the seasonal 2009-2010 nonadjuvanted Influenza virus vaccine in HIV-infected children, adolescents, and young adults in a randomized controlled trial.Clin Vaccine Immunol. 2011 Sep;18(9):1503-9. doi: 10.1128/CVI.05200-11. Epub 2011 Jul 27. Clin Vaccine Immunol. 2011. PMID: 21795458 Free PMC article. Clinical Trial.
-
Changes in antigen-specific IgG1 Fc N-glycosylation upon influenza and tetanus vaccination.Mol Cell Proteomics. 2012 Apr;11(4):M111.014563. doi: 10.1074/mcp.M111.014563. Epub 2011 Dec 19. Mol Cell Proteomics. 2012. PMID: 22184099 Free PMC article. Clinical Trial.
-
Higher antigen content improves the immune response to 2009 H1N1 influenza vaccine in HIV-infected adults: a randomized clinical trial.J Infect Dis. 2012 Mar 1;205(5):703-12. doi: 10.1093/infdis/jir837. Epub 2012 Jan 24. J Infect Dis. 2012. PMID: 22275399 Free PMC article. Clinical Trial.
References
-
- Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, et al. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58:1–207. - PubMed
-
- Neuzil KM, Coffey CS, Mitchel EF, Jr, Griffin MR. Cardiopulmonary hospitalizations during influenza season in adults and adolescents with advanced HIV infection. J Acquir Immune Defic Syndr. 2003;34:304–7. - PubMed
-
- Anema A, Mills E, Montaner J, Brownstein JS, Cooper C. Efficacy of influenza vaccination in HIV-positive patients: a systematic review and meta-analysis. HIV Med. 2008;9:57–61. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

