Opioid facilitation of rewarding electrical brain stimulation is suppressed in rats with neuropathic pain
- PMID: 21293250
- PMCID: PMC3073684
- DOI: 10.1097/ALN.0b013e31820a4edb
Opioid facilitation of rewarding electrical brain stimulation is suppressed in rats with neuropathic pain
Abstract
Introduction: Opioids are powerful analgesics, but are also common drugs of abuse. Few studies have examined how neuropathic pain alters the pharmacology of opioids in modulating limbic pathways that underlie abuse liability.
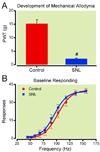
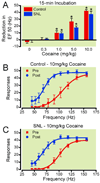
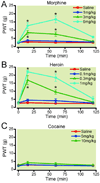
Methods: Rats with or without spinal nerve ligation (SNL) were implanted with electrodes into the left ventral tegmental area and trained to lever press for electrical stimulation. The effects of morphine, heroin, and cocaine on facilitating electrical stimulation of the ventral tegmental area and mechanical allodynia were assessed in SNL and control subjects.
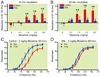
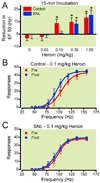
Results: Responding for electrical stimulation of the ventral tegmental area was similar in control and SNL rats. The frequency at which rats emitted 50% of maximal responding was 98.2 ± 5.1 (mean ± SEM) and 93.7 ± 2.8 Hz in control and SNL rats, respectively. Morphine reduced the frequency at which rats emitted 50% of maximal responding in control (maximal shift of 14.8 ± 3.1 Hz), but not SNL (2.3 ± 2.2 Hz) rats. Heroin was less potent in SNL rats, whereas cocaine produced similar shifts in control (42.3 ± 2.0 Hz) and SNL (37.5 ± 4.2 Hz) rats.
Conclusions: Nerve injury suppressed potentiation of electrical stimulation of the ventral tegmental area by opioids, suggesting that the positive reinforcing effects are diminished by chronic pain. Given concerns regarding prescription opioid abuse, developing strategies that assess both analgesia and abuse liability within the context of chronic pain may aid in determining which opioids are most suitable for treating chronic pain when abuse is a concern.
Figures
Comment in
-
When chronic pain can suppress the opioid kick: new insights from preclinical research.Anesthesiology. 2011 Mar;114(3):493-4. doi: 10.1097/ALN.0b013e31820a4ef7. Anesthesiology. 2011. PMID: 21278566 No abstract available.
Similar articles
-
Rewarding electrical brain stimulation in rats after peripheral nerve injury: decreased facilitation by commonly abused prescription opioids.Anesthesiology. 2011 Dec;115(6):1271-80. doi: 10.1097/ALN.0b013e3182330448. Anesthesiology. 2011. PMID: 21946150 Free PMC article.
-
Intracranial self-stimulation of the paraventricular nucleus of the hypothalamus: increased faciliation by morphine compared to cocaine.Anesthesiology. 2012 May;116(5):1116-23. doi: 10.1097/ALN.0b013e3182518be3. Anesthesiology. 2012. PMID: 22421421 Free PMC article.
-
Differential suppression of intracranial self-stimulation, food-maintained operant responding, and open field activity by paw incision and spinal nerve ligation in rats.Anesth Analg. 2014 Apr;118(4):854-62. doi: 10.1213/ANE.0000000000000119. Anesth Analg. 2014. PMID: 24651240
-
Determinants of opioid abuse potential: Insights using intracranial self-stimulation.Peptides. 2019 Feb;112:23-31. doi: 10.1016/j.peptides.2018.10.007. Epub 2018 Nov 1. Peptides. 2019. PMID: 30391425 Free PMC article. Review.
-
The Dopaminergic System in the Ventral Tegmental Area Contributes to Morphine Analgesia and Tolerance.Neuroscience. 2023 Sep 1;527:74-83. doi: 10.1016/j.neuroscience.2023.05.026. Epub 2023 Jun 5. Neuroscience. 2023. PMID: 37286162 Review.
Cited by
-
Fentanyl: Receptor pharmacology, abuse potential, and implications for treatment.Neurosci Biobehav Rev. 2019 Nov;106:49-57. doi: 10.1016/j.neubiorev.2018.12.005. Epub 2018 Dec 5. Neurosci Biobehav Rev. 2019. PMID: 30528374 Free PMC article. Review.
-
Analgesics as reinforcers with chronic pain: Evidence from operant studies.Neurosci Lett. 2013 Dec 17;557 Pt A(0 0):60-4. doi: 10.1016/j.neulet.2013.08.018. Epub 2013 Aug 22. Neurosci Lett. 2013. PMID: 23973302 Free PMC article. Review.
-
Effects of paclitaxel on mechanical sensitivity and morphine reward in male and female C57Bl6 mice.Exp Clin Psychopharmacol. 2016 Dec;24(6):485-495. doi: 10.1037/pha0000097. Exp Clin Psychopharmacol. 2016. PMID: 27929349 Free PMC article.
-
Chronic inflammatory pain promotes place preference for fentanyl in male rats but does not change fentanyl self-administration in male and female rats.Neuropharmacology. 2023 Jun 15;231:109512. doi: 10.1016/j.neuropharm.2023.109512. Epub 2023 Mar 21. Neuropharmacology. 2023. PMID: 36948356 Free PMC article.
-
Rewarding electrical brain stimulation in rats after peripheral nerve injury: decreased facilitation by commonly abused prescription opioids.Anesthesiology. 2011 Dec;115(6):1271-80. doi: 10.1097/ALN.0b013e3182330448. Anesthesiology. 2011. PMID: 21946150 Free PMC article.
References
-
- Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: Concerns and strategies. Drug Alcohol Depend. 2006;81:103–107. - PubMed
-
- Benedetti F, Vighetti S, Amanzio M, Casadio C, Oliaro A, Bergamasco B, Maggi G. Dose-response relationship of opioids in nociceptive and neuropathic postoperative pain. Pain. 1998;74:205–211. - PubMed
-
- Martin TJ, Eisenach JC. Pharmacology of opioid and nonopioid analgesics in chronic pain states. J Pharmacol Exp Ther. 2001;299:811–817. - PubMed
-
- Martin TJ, Kim SA, Buechler NL, Porreca F, Eisenach JC. Opioid self-administration in the nerve-injured rat: Relevance of antiallodynic effects to drug consumption and effects of intrathecal analgesics. Anesthesiology. 2007;106:312–322. - PubMed
-
- Ozaki S, Narita M, Iino M, Sugita J, Matsumura Y, Suzuki T. Suppression of the morphine-induced rewarding effect in the rat with neuropathic pain: Implication of the reduction in mu-opioid receptor functions in the ventral tegmental area. J Neurochem. 2002;82:1192–1198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

