Subregional specification of embryonic stem cell-derived ventral telencephalic tissues by timed and combinatory treatment with extrinsic signals
- PMID: 21289201
- PMCID: PMC6623725
- DOI: 10.1523/JNEUROSCI.5128-10.2011
Subregional specification of embryonic stem cell-derived ventral telencephalic tissues by timed and combinatory treatment with extrinsic signals
Abstract
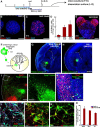
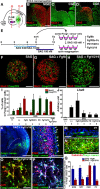
During early telencephalic development, the major portion of the ventral telencephalic (subpallial) region becomes subdivided into three regions, the lateral (LGE), medial (MGE), and caudal (CGE) ganglionic eminences. In this study, we systematically recapitulated subpallial patterning in mouse embryonic stem cell (ESC) cultures and investigated temporal and combinatory actions of patterning signals. In serum-free floating culture, the dorsal-ventral specification of ESC-derived telencephalic neuroectoderm is dose-dependently directed by Sonic hedgehog (Shh) signaling. Early Shh treatment, even before the expression onset of Foxg1 (also Bf1; earliest marker of the telencephalic lineage), is critical for efficiently generating LGE progenitors, and continuous Shh signaling until day 9 is necessary to commit these cells to the LGE lineage. When induced under these conditions and purified by fluorescence-activated cell sorter, telencephalic cells efficiently differentiated into Nolz1(+)/Ctip2(+) LGE neuronal precursors and subsequently, both in culture and after in vivo grafting, into DARPP32(+) medium-sized spiny neurons. Purified telencephalic progenitors treated with high doses of the Hedgehog (Hh) agonist SAG (Smoothened agonist) differentiated into MGE- and CGE-like tissues. Interestingly, in addition to strong Hh signaling, the efficient specification of MGE cells requires Fgf8 signaling but is inhibited by treatment with Fgf15/19. In contrast, CGE differentiation is promoted by Fgf15/19 but suppressed by Fgf8, suggesting that specific Fgf signals play different, critical roles in the positional specification of ESC-derived ventral subpallial tissues. We discuss a model of the antagonistic Fgf8 and Fgf15/19 signaling in rostral-caudal subpallial patterning and compare it with the roles of these molecules in cortical patterning.
Figures
Similar articles
-
The transcription factor Foxg1 regulates the competence of telencephalic cells to adopt subpallial fates in mice.Development. 2010 Feb;137(3):487-97. doi: 10.1242/dev.039800. Development. 2010. PMID: 20081193 Free PMC article.
-
Pharmacological modulation of the Hedgehog pathway differentially affects dorsal/ventral patterning in mouse and human embryonic stem cell models of telencephalic development.Stem Cells Dev. 2012 May 1;21(7):1016-46. doi: 10.1089/scd.2011.0271. Epub 2012 Feb 8. Stem Cells Dev. 2012. PMID: 22204396
-
FGF15 promotes neurogenesis and opposes FGF8 function during neocortical development.Neural Dev. 2008 Jul 14;3:17. doi: 10.1186/1749-8104-3-17. Neural Dev. 2008. PMID: 18625063 Free PMC article.
-
Sonic hedgehog functions through dynamic changes in temporal competence in the developing forebrain.Curr Opin Genet Dev. 2010 Aug;20(4):391-9. doi: 10.1016/j.gde.2010.04.008. Epub 2010 May 11. Curr Opin Genet Dev. 2010. PMID: 20466536 Free PMC article. Review.
-
Genetic patterning of the mammalian telencephalon by morphogenetic molecules and transcription factors.Birth Defects Res C Embryo Today. 2006 Sep;78(3):256-66. doi: 10.1002/bdrc.20077. Birth Defects Res C Embryo Today. 2006. PMID: 17061260 Review.
Cited by
-
Losing your inhibition: linking cortical GABAergic interneurons to schizophrenia.Neurobiol Dis. 2013 May;53:36-48. doi: 10.1016/j.nbd.2012.11.013. Epub 2012 Nov 29. Neurobiol Dis. 2013. PMID: 23201207 Free PMC article. Review.
-
Pax6 limits the competence of developing cerebral cortical cells to respond to inductive intercellular signals.PLoS Biol. 2022 Sep 6;20(9):e3001563. doi: 10.1371/journal.pbio.3001563. eCollection 2022 Sep. PLoS Biol. 2022. PMID: 36067211 Free PMC article.
-
Pluripotent stem cells for the study of CNS development.Front Mol Neurosci. 2011 Oct 12;4:30. doi: 10.3389/fnmol.2011.00030. eCollection 2011. Front Mol Neurosci. 2011. PMID: 22016722 Free PMC article.
-
Modeling of Autism Using Organoid Technology.Mol Neurobiol. 2017 Dec;54(10):7789-7795. doi: 10.1007/s12035-016-0274-8. Epub 2016 Nov 14. Mol Neurobiol. 2017. PMID: 27844287 Review.
-
CRISPR/Cas9-Correctable mutation-related molecular and physiological phenotypes in iPSC-derived Alzheimer's PSEN2 N141I neurons.Acta Neuropathol Commun. 2017 Oct 27;5(1):77. doi: 10.1186/s40478-017-0475-z. Acta Neuropathol Commun. 2017. PMID: 29078805 Free PMC article.
References
-
- Agoston DV, Szemes M, Dobi A, Palkovits M, Georgopoulos K, Gyorgy A, Ring MA. Ikaros is expressed in developing striatal neurons and involved in enkephalinergic differentiation. J Neurochem. 2007;102:1805–1816. - PubMed
-
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. - PubMed
-
- Anderson SA, Marín O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
