CD43 interaction with ezrin-radixin-moesin (ERM) proteins regulates T-cell trafficking and CD43 phosphorylation
- PMID: 21289089
- PMCID: PMC3069020
- DOI: 10.1091/mbc.E10-07-0586
CD43 interaction with ezrin-radixin-moesin (ERM) proteins regulates T-cell trafficking and CD43 phosphorylation
Abstract
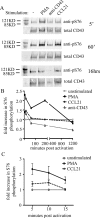
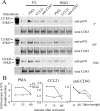
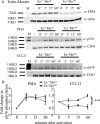
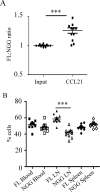
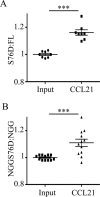
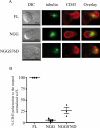
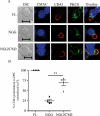
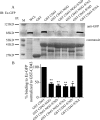
Cell polarization is a key feature of cell motility, driving cell migration to tissues. CD43 is an abundantly expressed molecule on the T-cell surface that shows distinct localization to the migrating T-cell uropod and the distal pole complex (DPC) opposite the immunological synapse via association with the ezrin-radixin-moesin (ERM) family of actin regulatory proteins. CD43 regulates multiple T-cell functions, including T-cell activation, proliferation, apoptosis, and migration. We recently demonstrated that CD43 regulates T-cell trafficking through a phosphorylation site at Ser-76 (S76) within its cytoplasmic tail. Using a phosphorylation-specific antibody, we now find that CD43 phosphorylation at S76 is enhanced by migration signals. We further show that CD43 phosphorylation and normal T-cell trafficking depend on CD43 association with ERM proteins. Interestingly, mutation of S76 to mimic phosphorylation enhances T-cell migration and CD43 movement to the DPC while blocking ERM association, showing that CD43 movement can occur in the absence of ERM binding. We also find that protein kinase CΘ can phosphorylate CD43. These results show that while CD43 binding to ERM proteins is crucial for S76 phosphorylation, CD43 movement and regulation of T-cell migration can occur through an ERM-independent, phosphorylation-dependent mechanism.
Figures
Similar articles
-
PKCθ regulates T cell motility via ezrin-radixin-moesin localization to the uropod.PLoS One. 2013 Nov 8;8(11):e78940. doi: 10.1371/journal.pone.0078940. eCollection 2013. PLoS One. 2013. PMID: 24250818 Free PMC article.
-
CD43 interacts with moesin and ezrin and regulates its redistribution to the uropods of T lymphocytes at the cell-cell contacts.Blood. 1998 Jun 15;91(12):4632-44. Blood. 1998. PMID: 9616160
-
Formation of microvilli and phosphorylation of ERM family proteins by CD43, a potent inhibitor for cell adhesion: cell detachment is a potential cue for ERM phosphorylation and organization of cell morphology.Cell Adh Migr. 2011 Mar-Apr;5(2):119-32. doi: 10.4161/cam.5.2.13908. Epub 2011 Mar 1. Cell Adh Migr. 2011. PMID: 21045567 Free PMC article.
-
ERM Proteins at the Crossroad of Leukocyte Polarization, Migration and Intercellular Adhesion.Int J Mol Sci. 2020 Feb 22;21(4):1502. doi: 10.3390/ijms21041502. Int J Mol Sci. 2020. PMID: 32098334 Free PMC article. Review.
-
Re-defining ERM function in lymphocyte activation and migration.Immunol Rev. 2013 Nov;256(1):63-79. doi: 10.1111/imr.12104. Immunol Rev. 2013. PMID: 24117813 Review.
Cited by
-
PKCθ regulates T cell motility via ezrin-radixin-moesin localization to the uropod.PLoS One. 2013 Nov 8;8(11):e78940. doi: 10.1371/journal.pone.0078940. eCollection 2013. PLoS One. 2013. PMID: 24250818 Free PMC article.
-
Transplantation elicits a clonally diverse CD8+ T cell response that is comprised of potent CD43+ effectors.Cell Rep. 2023 Aug 29;42(8):112993. doi: 10.1016/j.celrep.2023.112993. Epub 2023 Aug 16. Cell Rep. 2023. PMID: 37590141 Free PMC article.
-
Common polymorphisms in the CD43 gene region are associated with tuberculosis disease and mortality.Am J Respir Cell Mol Biol. 2015 Mar;52(3):342-8. doi: 10.1165/rcmb.2014-0114OC. Am J Respir Cell Mol Biol. 2015. PMID: 25078322 Free PMC article.
-
Mechanism of isorhynchophylline in lipopolysaccharide-induced acute lung injury based on proteomic technology.Front Pharmacol. 2024 May 30;15:1397498. doi: 10.3389/fphar.2024.1397498. eCollection 2024. Front Pharmacol. 2024. PMID: 38873411 Free PMC article.
-
Ezrin and moesin are required for efficient T cell adhesion and homing to lymphoid organs.PLoS One. 2013;8(2):e52368. doi: 10.1371/journal.pone.0052368. Epub 2013 Feb 28. PLoS One. 2013. PMID: 23468835 Free PMC article.
References
-
- Allenspach EJ, Cullinan P, Tong J, Tang Q, Tesciuba AG, Cannon JL, Takahashi SM, Morgan R, Burkhardt JK, Sperling AI. ERM–dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity. 2001;15:739–750. - PubMed
-
- Andersson CX, Fernandez-Rodriguez J, Laos S, Sikut R, Sikut A, Baeckstrom D, Hansson GC. CD43 has a functional NLS, interacts with β-catenin, and affects gene expression. Biochem Biophys Res Commun. 2004;316:12–17. - PubMed
-
- Brown MJ, Nijhara R, Hallam JA, Gignac M, Yamada KM, Erlandsen SL, Delon J, Kruhlak M, Shaw S. Chemokine stimulation of human peripheral blood T lymphocytes induces rapid dephosphorylation of ERM proteins, which facilitates loss of microvilli and polarization. Blood. 2003;102:3890–3899. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

