Ploidy and the evolution of parasitism
- PMID: 21288940
- PMCID: PMC3145178
- DOI: 10.1098/rspb.2010.2146
Ploidy and the evolution of parasitism
Abstract
Levels of parasitism are continuously distributed in nature. Models of host-parasite coevolution, however, typically assume that species can be easily characterized as either parasitic or non-parasitic. Consequently, it is poorly understood which factors influence the evolution of parasitism itself. We investigate how ploidy level and the genetic mechanisms underlying infection influence evolution along the continuum of parasitism levels. In order for parasitism to evolve, selective benefits to the successful invasion of hosts must outweigh the losses when encountering resistant hosts. However, we find that exactly where this threshold occurs depends not only on the strength of selection, but also on the genetic model of interaction, the ploidy level in each species, and the nature of the costs to virulence and resistance. With computer simulations, we are able to incorporate more realistic dynamics at the loci underlying species interactions and to extend our analyses in a number of directions, including finite population sizes, multiple alleles and different generation times.
This journal is © 2011 The Royal Society
Figures
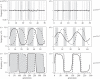
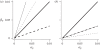

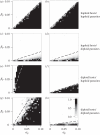
Similar articles
-
Host-parasite coevolution in a multilocus gene-for-gene system.Proc Biol Sci. 2000 Nov 7;267(1458):2183-8. doi: 10.1098/rspb.2000.1267. Proc Biol Sci. 2000. PMID: 11413631 Free PMC article.
-
Host-parasite interactions and the evolution of ploidy.Proc Natl Acad Sci U S A. 2004 Jul 27;101(30):11036-9. doi: 10.1073/pnas.0403151101. Epub 2004 Jul 13. Proc Natl Acad Sci U S A. 2004. PMID: 15252199 Free PMC article.
-
How parasite interaction strategies alter virulence evolution in multi-parasite communities.Evolution. 2019 Nov;73(11):2189-2203. doi: 10.1111/evo.13843. Epub 2019 Sep 24. Evolution. 2019. PMID: 31506940
-
Host-parasite coevolution: why changing population size matters.Zoology (Jena). 2016 Aug;119(4):330-8. doi: 10.1016/j.zool.2016.02.001. Epub 2016 Feb 12. Zoology (Jena). 2016. PMID: 27161157 Review.
-
Transmission modes and evolution of the parasitism-mutualism continuum.Ann N Y Acad Sci. 1987;503:295-306. doi: 10.1111/j.1749-6632.1987.tb40616.x. Ann N Y Acad Sci. 1987. PMID: 3304078 Review.
Cited by
-
Coevolutionary theory of hosts and parasites.J Evol Biol. 2022 Feb;35(2):205-224. doi: 10.1111/jeb.13981. Epub 2022 Jan 30. J Evol Biol. 2022. PMID: 35030276 Free PMC article. Review.
-
The evolution of mutation rate in an antagonistic coevolutionary model with maternal transmission of parasites.Proc Biol Sci. 2013 May 1;280(1761):20130647. doi: 10.1098/rspb.2013.0647. Print 2013 Jun 22. Proc Biol Sci. 2013. PMID: 23760645 Free PMC article.
-
Mutational effects depend on ploidy level: all else is not equal.Biol Lett. 2013 Feb 23;9(1):20120614. doi: 10.1098/rsbl.2012.0614. Epub 2012 Oct 10. Biol Lett. 2013. PMID: 23054913 Free PMC article.
References
-
- Frank S. A. 2002. Immunology and the evolution of infectious diseases. Princeton, NJ: Princeton University Press - PubMed
-
- Galvani A. P. 2003. Epidemiology meets evolutionary ecology. Trends Ecol. Evol. 18, 132–13910.1016/S0169-5347(02)00050-2 (doi:10.1016/S0169-5347(02)00050-2) - DOI - DOI
-
- May R. M., Anderson R. M. 1983. Epidemiology and genetics in the coevolution of parasites and hosts. Proc. R. Soc. Lond. B 219, 281–31310.1098/rspb.1983.0075 (doi:10.1098/rspb.1983.0075) - DOI - DOI - PubMed
-
- Hamilton W. D., Axelrod R., Tanese R. 1990. Sexual reproduction as an adaptation to resist parasites (a review). Proc. Natl Acad. Sci. USA 87, 3566–357310.1073/pnas.87.9.3566 (doi:10.1073/pnas.87.9.3566) - DOI - DOI - PMC - PubMed
-
- Peters A. D., Lively C. M. 2007. Short- and long-term benefits and detriments to recombination under antagonistic coevolution. J. Evol. Biol. 20, 1206–121710.1111/j.1420-9101.2006.01283.x (doi:10.1111/j.1420-9101.2006.01283.x) - DOI - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

