Role of innate immunity in a murine model of histidyl-transfer RNA synthetase (Jo-1)-mediated myositis
- PMID: 21280002
- PMCID: PMC3035731
- DOI: 10.1002/art.30113
Role of innate immunity in a murine model of histidyl-transfer RNA synthetase (Jo-1)-mediated myositis
Abstract
Objective: Previous studies in humans and in animal models support a key role of histidyl-transfer RNA synthetase (HisRS; also known as Jo-1) in the pathogenesis of idiopathic inflammatory myopathy. While most investigations have focused on the ability of HisRS to trigger adaptive immune responses, in vitro studies clearly indicate that HisRS possesses intrinsic chemokine-like properties capable of activating the innate immune system. The purpose of this study was therefore to examine the ability of HisRS to direct innate immune responses in a murine model of myositis.
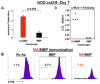
Methods: Following intramuscular immunization with soluble HisRS in the absence of exogenous adjuvant, selected strains of mice were evaluated at different time points for histopathologic evidence of myositis. Enzyme-linked immunosorbent assay-based assessment of autoantibody formation and carboxyfluorescein succinimidyl ester proliferation studies provided complementary measures of B cell and T cell responses triggered by HisRS immunization.
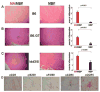
Results: Compared to appropriate control proteins, a murine HisRS fusion protein induced robust, statistically significant muscle inflammation in multiple congenic strains of C57BL/6 and NOD mice. Time course experiments revealed that this inflammatory response occurred as early as 7 days postimmunization and persisted for up to 7 weeks. Parallel immunization strategies in DO11.10/RAG-2(-/-) and C3H/HeJ (TLR-4(-/-) ) mice indicated that the ability of murine HisRS to drive muscle inflammation was not dependent on B cell receptor or T cell receptor recognition and did not require Toll-like receptor 4 signaling.
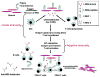
Conclusion: Collectively, the findings of these experiments support a model in which HisRS can trigger both innate and adaptive immune responses that culminate in severe muscle inflammation that is the hallmark of idiopathic inflammatory myopathy.
Copyright © 2011 by the American College of Rheumatology.
Figures



Comment in
-
Histidyl-transfer RNA synthetase: a key participant in idiopathic inflammatory myopathies.Arthritis Rheum. 2011 Feb;63(2):331-3. doi: 10.1002/art.30110. Arthritis Rheum. 2011. PMID: 21279988 No abstract available.
Similar articles
-
Role of Jo-1 in the Immunopathogenesis of the Anti-synthetase Syndrome.Curr Rheumatol Rep. 2015 Sep;17(9):56. doi: 10.1007/s11926-015-0532-1. Curr Rheumatol Rep. 2015. PMID: 26210509 Review.
-
Antigen-driven T cell-macrophage interactions mediate the interface between innate and adaptive immunity in histidyl-tRNA synthetase-induced myositis.Front Immunol. 2023 Sep 22;14:1238221. doi: 10.3389/fimmu.2023.1238221. eCollection 2023. Front Immunol. 2023. PMID: 37809058 Free PMC article.
-
Required role of apoptotic myogenic precursors and toll-like receptor stimulation for the establishment of autoimmune myositis in experimental murine models.Arthritis Rheumatol. 2015 Mar;67(3):809-22. doi: 10.1002/art.38985. Arthritis Rheumatol. 2015. PMID: 25504878
-
Novel conformation of histidyl-transfer RNA synthetase in the lung: the target tissue in Jo-1 autoantibody-associated myositis.Arthritis Rheum. 2007 Aug;56(8):2729-39. doi: 10.1002/art.22790. Arthritis Rheum. 2007. PMID: 17665459
-
Immunopathogenesis of the Anti-Synthetase Syndrome.Crit Rev Immunol. 2018;38(4):263-278. doi: 10.1615/CritRevImmunol.2018025744. Crit Rev Immunol. 2018. PMID: 30806243 Review.
Cited by
-
Antisynthetase syndrome.Curr Rheumatol Rep. 2011 Jun;13(3):175-81. doi: 10.1007/s11926-011-0176-8. Curr Rheumatol Rep. 2011. PMID: 21455765 Review.
-
Role of Jo-1 in the Immunopathogenesis of the Anti-synthetase Syndrome.Curr Rheumatol Rep. 2015 Sep;17(9):56. doi: 10.1007/s11926-015-0532-1. Curr Rheumatol Rep. 2015. PMID: 26210509 Review.
-
Antigen-driven T cell-macrophage interactions mediate the interface between innate and adaptive immunity in histidyl-tRNA synthetase-induced myositis.Front Immunol. 2023 Sep 22;14:1238221. doi: 10.3389/fimmu.2023.1238221. eCollection 2023. Front Immunol. 2023. PMID: 37809058 Free PMC article.
-
Active JNK-dependent secretion of Drosophila Tyrosyl-tRNA synthetase by loser cells recruits haemocytes during cell competition.Nat Commun. 2015 Dec 11;6:10022. doi: 10.1038/ncomms10022. Nat Commun. 2015. PMID: 26658841
-
Activation of autoreactive B cells by endogenous TLR7 and TLR3 RNA ligands.J Biol Chem. 2012 Nov 16;287(47):39789-99. doi: 10.1074/jbc.M112.383000. Epub 2012 Sep 27. J Biol Chem. 2012. PMID: 23019335 Free PMC article.
References
-
- Greenberg SA. Inflammatory myopathies: disease mechanisms. Curr Opin Neurol. 2009;22:516–23. - PubMed
-
- Suber TL, Casciola-Rosen L, Rosen A. Mechanisms of disease: autoantigens as clues to the pathogenesis of myositis. Nat Clin Pract Rheumatol. 2008;4:201–9. - PubMed
-
- Pignone A, Fiori G, Del Rosso A, Generini S, Matucci-Cerinic M. The pathogenesis of inflammatory muscle diseases: on the cutting edge among the environment, the genetic background, the immune response and the dysregulation of apoptosis. Autoimmun Rev. 2002;1:226–32. - PubMed
-
- Katsumata Y, Ascherman DP. Animal models in myositis. Curr Opin Rheumatol. 2008;20:681–5. - PubMed
-
- Nagaraju K, Plotz PH. Animal models of myositis. Rheum Dis Clin North Am. 2002;28:917–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

