The pathological roles of ganglioside metabolism in Alzheimer's disease: effects of gangliosides on neurogenesis
- PMID: 21274438
- PMCID: PMC3025365
- DOI: 10.4061/2011/193618
The pathological roles of ganglioside metabolism in Alzheimer's disease: effects of gangliosides on neurogenesis
Abstract
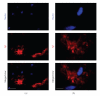
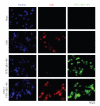
Conversion of the soluble, nontoxic amyloid β-protein (Aβ) into an aggregated, toxic form rich in β-sheets is a key step in the onset of Alzheimer's disease (AD). It has been suggested that Aβ induces changes in neuronal membrane fluidity as a result of its interactions with membrane components such as cholesterol, phospholipids, and gangliosides. Gangliosides are known to bind Aβ. A complex of GM1 and Aβ, termed "GAβ", has been identified in AD brains. Abnormal ganglioside metabolism also may occur in AD brains. We have reported an increase of Chol-1α antigens, GQ1bα and GT1aα, in the brain of transgenic mouse AD model. GQ1bα and GT1aα exhibit high affinities to Aβs. The presence of Chol-1α gangliosides represents evidence for genesis of cholinergic neurons in AD brains. We evaluated the effects of GM1 and Aβ1-40 on mouse neuroepithelial cells. Treatment of these cells simultaneously with GM1 and Aβ1-40 caused a significant reduction of cell number, suggesting that Aβ1-40 and GM1 cooperatively exert a cytotoxic effect on neuroepithelial cells. An understanding of the mechanism on the interaction of GM1 and Aβs in AD may contribute to the development of new neuroregenerative therapies for this disorder.
Figures

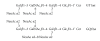
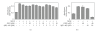
Similar articles
-
Ganglioside metabolism in a transgenic mouse model of Alzheimer's disease: expression of Chol-1α antigens in the brain.ASN Neuro. 2010 Oct 4;2(4):e00044. doi: 10.1042/AN20100021. ASN Neuro. 2010. PMID: 20930939 Free PMC article.
-
Brain Gangliosides in Alzheimer's Disease: Increased Expression of Cholinergic Neuron-Specific Gangliosides.Curr Alzheimer Res. 2017;14(6):586-591. doi: 10.2174/1567205014666170117094038. Curr Alzheimer Res. 2017. PMID: 28124591
-
The Pathogenic Role of Ganglioside Metabolism in Alzheimer's Disease-Cholinergic Neuron-Specific Gangliosides and Neurogenesis.Mol Neurobiol. 2017 Jan;54(1):623-638. doi: 10.1007/s12035-015-9641-0. Mol Neurobiol. 2017. PMID: 26748510 Review.
-
Role of ganglioside metabolism in the pathogenesis of Alzheimer's disease--a review.J Lipid Res. 2008 Jun;49(6):1157-75. doi: 10.1194/jlr.R800007-JLR200. Epub 2008 Mar 11. J Lipid Res. 2008. PMID: 18334715 Free PMC article. Review.
-
Ganglioside-Dependent Neural Stem Cell Proliferation in Alzheimer's Disease Model Mice.ASN Neuro. 2015 Dec 23;7(6):1759091415618916. doi: 10.1177/1759091415618916. Print 2015 Nov-Dec. ASN Neuro. 2015. PMID: 26699276 Free PMC article.
Cited by
-
Spin-labeling Insights into How Chemical Fixation Impacts Glycan Organization on Cells.Appl Magn Reson. 2024 Mar;55(1-3):317-333. doi: 10.1007/s00723-023-01624-w. Epub 2023 Oct 9. Appl Magn Reson. 2024. PMID: 38469359 Free PMC article.
-
Functional roles of gangliosides in neurodevelopment: an overview of recent advances.Neurochem Res. 2012 Jun;37(6):1230-44. doi: 10.1007/s11064-012-0744-y. Epub 2012 Mar 13. Neurochem Res. 2012. PMID: 22410735 Free PMC article. Review.
-
The role of APP proteolytic processing in lipid metabolism.Exp Brain Res. 2012 Apr;217(3-4):365-75. doi: 10.1007/s00221-011-2975-6. Epub 2011 Dec 17. Exp Brain Res. 2012. PMID: 22179528 Review.
-
Delineating Amyloid Plaque Associated Neuronal Sphingolipids in Transgenic Alzheimer's Disease Mice (tgArcSwe) Using MALDI Imaging Mass Spectrometry.ACS Chem Neurosci. 2017 Feb 15;8(2):347-355. doi: 10.1021/acschemneuro.6b00391. Epub 2017 Jan 10. ACS Chem Neurosci. 2017. PMID: 27984697 Free PMC article.
-
Sialometabolism in Brain Health and Alzheimer's Disease.Front Neurosci. 2021 Mar 30;15:648617. doi: 10.3389/fnins.2021.648617. eCollection 2021. Front Neurosci. 2021. PMID: 33867926 Free PMC article. Review.
References
-
- Ariga T, Miyatake T, Yu RK. Role of proteoglycans and glycosaminoglycans in the pathogenesis of Alzheimer's disease and related disorders: amyloidogenesis and therapeutic strategies—a review. Journal of Neuroscience Research. 2010;88(11):2303–2315. - PubMed
-
- Ngamukote S, Yanagisawa M, Ariga T, Ando S, Yu RK. Developmental changes of glycosphingolipids and expression of glycogenes in mouse brains. Journal of Neurochemistry. 2007;103(6):2327–2341. - PubMed
-
- Yanagisawa M, Yu RK. The expression and functions of glycoconjugates in neural stem cells. Glycobiology. 2007;17(7):57R–74R. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

