Aldosterone stimulates superoxide production in macula densa cells
- PMID: 21270097
- PMCID: PMC3174554
- DOI: 10.1152/ajprenal.00596.2010
Aldosterone stimulates superoxide production in macula densa cells
Abstract
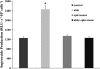
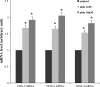
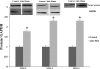
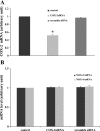
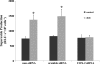
Two major factors which regulate tubuloglomerular feedback (TGF)-mediated constriction of the afferent arteriole are release of superoxide (O(2)(-)) and nitric oxide (NO) by macula densa (MD) cells. MD O(2)(-) inactivates NO; however, among the factors that increase MD O(2)(-) release, the role of aldosterone is unclear. We hypothesize that aldosterone activates the mineralocorticoid receptor (MR) on MD cells, resulting in increased O(2)(-) production due to upregulation of cyclooxygenase-1 (COX-2) and NOX-2, and NOX-4, isoforms of NAD(P)H oxidase. Studies were performed on MMDD1 cells, a renal epithelial cell line with properties of MD cells. RT-PCR and Western blotting confirmed the expression of MR. Aldosterone (10(-8) mol/l for 30 min) doubled MMDD1 cell O(2)(-) production, and this was completely blocked by MR inhibition with 10(-5) mol/l eplerenone. RT-PCR, real-time PCR, and Western blotting demonstrated aldosterone-induced increases in COX-2, NOX-2, and NOX-4 expression. Inhibition of COX-2 (NS398), NADPH oxidase (apocynin), or a combination blocked aldosterone-induced O(2)(-) production to the same degree. These data suggest that aldosterone-stimulated MD O(2)(-) production is mediated by COX-2 and NADPH oxidase. Next, COX-2 small-interfering RNA (siRNA) specifically decreased COX-2 mRNA without affecting NOX-2 or NOX-4 mRNAs. In the presence of the COX-2 siRNA, the aldosterone-induced increases in COX-2, NOX-2, and NOX-4 mRNAs and O(2)(-) production were completely blocked, suggesting that COX-2 causes increased expression of NOX-2 and NOX-4. In conclusion 1) MD cells express MR; 2) aldosterone increases O(2)(-) production by activating MR; and 3) aldosterone stimulates COX-2, which further activates NOX-2 and NOX-4 and generates O(2)(-). The resulting balance between O(2)(-) and NO in the MD is important in modulating TGF.
Figures


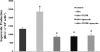
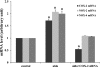
Similar articles
-
NOX2 is the primary source of angiotensin II-induced superoxide in the macula densa.Am J Physiol Regul Integr Comp Physiol. 2010 Mar;298(3):R707-12. doi: 10.1152/ajpregu.00762.2009. Epub 2010 Jan 6. Am J Physiol Regul Integr Comp Physiol. 2010. PMID: 20053956 Free PMC article.
-
Interaction between nitric oxide and superoxide in the macula densa in aldosterone-induced alterations of tubuloglomerular feedback.Am J Physiol Renal Physiol. 2013 Feb 1;304(3):F326-32. doi: 10.1152/ajprenal.00501.2012. Epub 2012 Dec 5. Am J Physiol Renal Physiol. 2013. PMID: 23220724 Free PMC article.
-
Enhanced expression and activity of Nox2 and Nox4 in the macula densa in ANG II-induced hypertensive mice.Am J Physiol Renal Physiol. 2014 Feb 1;306(3):F344-50. doi: 10.1152/ajprenal.00515.2013. Epub 2013 Nov 27. Am J Physiol Renal Physiol. 2014. PMID: 24285500 Free PMC article.
-
Cyclooxygenase-2 and the renal renin-angiotensin system.Acta Physiol Scand. 2004 Aug;181(4):543-7. doi: 10.1111/j.1365-201X.2004.01329.x. Acta Physiol Scand. 2004. PMID: 15283769 Review.
-
NOX/NADPH oxidase, the superoxide-generating enzyme: its transcriptional regulation and physiological roles.J Pharmacol Sci. 2010;114(2):134-46. doi: 10.1254/jphs.10r01cr. Epub 2010 Sep 11. J Pharmacol Sci. 2010. PMID: 20838023 Review.
Cited by
-
Aldosterone blunts tubuloglomerular feedback by activating macula densa mineralocorticoid receptors.Hypertension. 2012 Mar;59(3):599-606. doi: 10.1161/HYPERTENSIONAHA.111.173195. Epub 2012 Feb 6. Hypertension. 2012. PMID: 22311906 Free PMC article.
-
Shear stress blunts tubuloglomerular feedback partially mediated by primary cilia and nitric oxide at the macula densa.Am J Physiol Regul Integr Comp Physiol. 2015 Oct;309(7):R757-66. doi: 10.1152/ajpregu.00173.2015. Epub 2015 Aug 12. Am J Physiol Regul Integr Comp Physiol. 2015. PMID: 26269519 Free PMC article.
-
Mechanisms of connecting tubule glomerular feedback enhancement by aldosterone.Am J Physiol Renal Physiol. 2016 Dec 1;311(6):F1182-F1188. doi: 10.1152/ajprenal.00076.2016. Epub 2016 Jul 13. Am J Physiol Renal Physiol. 2016. PMID: 27413197 Free PMC article.
-
What can urinary exosomes tell us?Cell Mol Life Sci. 2021 Apr;78(7):3265-3283. doi: 10.1007/s00018-020-03739-w. Epub 2021 Jan 28. Cell Mol Life Sci. 2021. PMID: 33507324 Free PMC article. Review.
-
Angiotensin and mineralocorticoid receptor antagonism attenuates cardiac oxidative stress in angiotensin II-infused rats.Clin Exp Pharmacol Physiol. 2015 Nov;42(11):1178-88. doi: 10.1111/1440-1681.12473. Clin Exp Pharmacol Physiol. 2015. PMID: 26234762 Free PMC article.
References
-
- Arima S, Kohagura K, Xu HL, Sugawara A, Abe T, Satoh F, Takeuchi K, Ito S. Nongenomic vascular action of aldosterone in the glomerular microcirculation. J Am Soc Nephrol 14: 2255–2263, 2003 - PubMed
-
- Beswick RA, Dorrance AM, Leite R, Webb RC. NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension 38: 1107–1111, 2001 - PubMed
-
- Blantz RC, Pelayo JC. A functional role for the tubuloglomerular feedback mechanism. Kidney Int 25: 739–746, 1984 - PubMed
-
- DeMarco VG, Habibi J, Whaley-Connell AT, Schneider RI, Heller RL, Bosanquet JP, Hayden MR, Delcour K, Cooper SA, Andresen BT, Sowers JR, Dellsperger KC. Oxidative stress contributes to pulmonary hypertension in the transgenic (mRen2)27 rat. Am J Physiol Heart Circ Physiol 294: H2659–H2668, 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

