BACE1 retrograde trafficking is uniquely regulated by the cytoplasmic domain of sortilin
- PMID: 21245145
- PMCID: PMC3069461
- DOI: 10.1074/jbc.M110.170217
BACE1 retrograde trafficking is uniquely regulated by the cytoplasmic domain of sortilin
Abstract
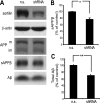
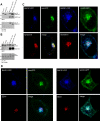
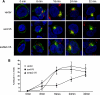
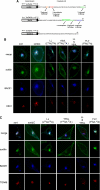
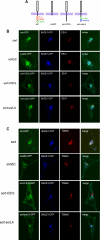
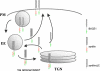
BACE1 (β-site β-amyloid precursor protein (APP)-cleaving enzyme 1) mediates the first proteolytic cleavage of APP, leading to amyloid β-peptide (Aβ) production. It has been reported that BACE1 intracellular trafficking, in particular endosome-to-TGN sorting, is mediated by adaptor complexes, such as retromer and Golgi-localized γ-ear-containing ARF-binding proteins (GGAs). Here we investigated whether sortilin, a Vps10p domain-sorting receptor believed to participate in retromer-mediated transport of select membrane cargoes, contributes to the subcellular trafficking and activity of BACE1. Our initial studies revealed increased levels of sortilin in post-mortem brain tissue of AD patients and that overexpression of sortilin leads to increased BACE1-mediated cleavage of APP in cultured cells. In contrast, RNAi suppression of sortilin results in decreased BACE1-mediated cleavage of APP. We also found that sortilin interacts with BACE1 and that a sortilin construct lacking its cytoplasmic domain, which contains putative retromer sorting motifs, remains bound to BACE1. However, expression of this truncated sortilin redistributes BACE1 from the trans-Golgi network to the endosomes and substantially reduces the retrograde trafficking of BACE1. Site-directed mutagenesis and chimera experiments reveal that the cytoplasmic tail of sortilin, but not those from other VPS10p domain receptors (e.g. SorCs1b and SorLA), plays a unique role in BACE1 trafficking. Our studies suggest a new function for sortilin as a modulator of BACE1 retrograde trafficking and subsequent generation of Aβ.
Figures

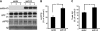

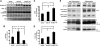
Similar articles
-
The Endosome-associated Deubiquitinating Enzyme USP8 Regulates BACE1 Enzyme Ubiquitination and Degradation.J Biol Chem. 2016 Jul 22;291(30):15753-66. doi: 10.1074/jbc.M116.718023. Epub 2016 Jun 14. J Biol Chem. 2016. PMID: 27302062 Free PMC article.
-
The Golgi-Localized γ-Ear-Containing ARF-Binding (GGA) Proteins Alter Amyloid-β Precursor Protein (APP) Processing through Interaction of Their GAE Domain with the Beta-Site APP Cleaving Enzyme 1 (BACE1).PLoS One. 2015 Jun 8;10(6):e0129047. doi: 10.1371/journal.pone.0129047. eCollection 2015. PLoS One. 2015. PMID: 26053850 Free PMC article.
-
Β-site APP-cleaving enzyme 1 trafficking and Alzheimer's disease pathogenesis.J Neurochem. 2012 Mar;120(6):869-80. doi: 10.1111/j.1471-4159.2011.07623.x. Epub 2012 Jan 23. J Neurochem. 2012. PMID: 22171895 Review.
-
GGA1-mediated endocytic traffic of LR11/SorLA alters APP intracellular distribution and amyloid-β production.Mol Biol Cell. 2012 Jul;23(14):2645-57. doi: 10.1091/mbc.E12-01-0014. Epub 2012 May 23. Mol Biol Cell. 2012. PMID: 22621900 Free PMC article.
-
Retromers in Alzheimer's disease.Neurodegener Dis. 2012;10(1-4):116-21. doi: 10.1159/000335910. Epub 2012 Mar 3. Neurodegener Dis. 2012. PMID: 22398391 Review.
Cited by
-
The Endosome-associated Deubiquitinating Enzyme USP8 Regulates BACE1 Enzyme Ubiquitination and Degradation.J Biol Chem. 2016 Jul 22;291(30):15753-66. doi: 10.1074/jbc.M116.718023. Epub 2016 Jun 14. J Biol Chem. 2016. PMID: 27302062 Free PMC article.
-
Regional and Cellular Mapping of Sortilin Immunoreactivity in Adult Human Brain.Front Neuroanat. 2019 Mar 12;13:31. doi: 10.3389/fnana.2019.00031. eCollection 2019. Front Neuroanat. 2019. PMID: 30914927 Free PMC article.
-
The understudied links of the retromer complex to age-related pathways.Geroscience. 2022 Feb;44(1):19-24. doi: 10.1007/s11357-021-00430-1. Epub 2021 Aug 9. Geroscience. 2022. PMID: 34370162 Free PMC article.
-
Trafficking in Alzheimer's Disease: Modulation of APP Transport and Processing by the Transmembrane Proteins LRP1, SorLA, SorCS1c, Sortilin, and Calsyntenin.Mol Neurobiol. 2018 Jul;55(7):5809-5829. doi: 10.1007/s12035-017-0806-x. Epub 2017 Oct 27. Mol Neurobiol. 2018. PMID: 29079999 Review.
-
Amyloid β-protein oligomers upregulate the β-secretase, BACE1, through a post-translational mechanism involving its altered subcellular distribution in neurons.Mol Brain. 2015 Nov 9;8(1):73. doi: 10.1186/s13041-015-0163-5. Mol Brain. 2015. PMID: 26552445 Free PMC article.
References
-
- Cummings J. L. (2004) N. Engl. J. Med. 351, 56–67 - PubMed
-
- Van Gassen G., Annaert W. (2003) Neuroscientist 9, 117–126 - PubMed
-
- Sinha S., Anderson J. P., Barbour R., Basi G. S., Caccavello R., Davis D., Doan M., Dovey H. F., Frigon N., Hong J., Jacobson-Croak K., Jewett N., Keim P., Knops J., Lieberburg I., Power M., Tan H., Tatsuno G., Tung J., Schenk D., Seubert P., Suomensaari S. M., Wang S., Walker D., Zhao J., McConlogue L., John V. (1999) Nature 402, 537–540 - PubMed
-
- Vassar R., Bennett B. D., Babu-Khan S., Kahn S., Mendiaz E. A., Denis P., Teplow D. B., Ross S., Amarante P., Loeloff R., Luo Y., Fisher S., Fuller J., Edenson S., Lile J., Jarosinski M. A., Biere A. L., Curran E., Burgess T., Louis J. C., Collins F., Treanor J., Rogers G., Citron M. (1999) Science 286, 735–741 - PubMed
-
- Walter J., Kaether C., Steiner H., Haass C. (2001) Curr. Opin. Neurobiol. 11, 585–590 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

