High-fat diet and glucocorticoid treatment cause hyperglycemia associated with adiponectin receptor alterations
- PMID: 21244702
- PMCID: PMC3031255
- DOI: 10.1186/1476-511X-10-11
High-fat diet and glucocorticoid treatment cause hyperglycemia associated with adiponectin receptor alterations
Abstract
Background: Adiponectin is the most abundant plasma protein synthesized for the most part in adipose tissue, and it is an insulin-sensitive hormone, playing a central role in glucose and lipid metabolism. In addition, it increases fatty acid oxidation in the muscle and potentiates insulin inhibition of hepatic gluconeogenesis. Two adiponectin receptors have been identified: AdipoR1 is the major receptor expressed in skeletal muscle, whereas AdipoR2 is mainly expressed in liver. Consumption of high levels of dietary fat is thought to be a major factor in the promotion of obesity and insulin resistance. Excessive levels of cortisol are characterized by the symptoms of abdominal obesity, hypertension, glucose intolerance or diabetes and dyslipidemia; of note, all of these features are shared by the condition of insulin resistance. Although it has been shown that glucocorticoids inhibit adiponectin expression in vitro and in vivo, little is known about the regulation of adiponectin receptors. The link between glucocorticoids and insulin resistance may involve the adiponectin receptors and adrenalectomy might play a role not only in regulate expression and secretion of adiponectin, as well regulate the respective receptors in several tissues.
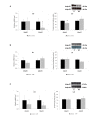
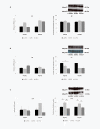
Results: Feeding of a high-fat diet increased serum glucose levels and decreased adiponectin and adipoR2 mRNA expression in subcutaneous and retroperitoneal adipose tissues, respectively. Moreover, it increased both adipoR1 and adipoR2 mRNA levels in muscle and adipoR2 protein levels in liver. Adrenalectomy combined with the synthetic glucocorticoid dexamethasone treatment resulted in increased glucose and insulin levels, decreased serum adiponectin levels, reduced adiponectin mRNA in epididymal adipose tissue, reduction of adipoR2 mRNA by 7-fold in muscle and reduced adipoR1 and adipoR2 protein levels in muscle. Adrenalectomy alone increased adiponectin mRNA expression 3-fold in subcutaneous adipose tissue and reduced adipoR2 mRNA expression 2-fold in liver.
Conclusion: Hyperglycemia as a result of a high-fat diet is associated with an increase in the expression of the adiponectin receptors in muscle. An excess of glucocorticoids, rather than their absence, increase glucose and insulin and decrease adiponectin levels.
Figures




Similar articles
-
Effects of adrenal hormones on the expression of adiponectin and adiponectin receptors in adipose tissue, muscle and liver.Steroids. 2011 Nov;76(12):1260-7. doi: 10.1016/j.steroids.2011.06.004. Epub 2011 Jun 30. Steroids. 2011. PMID: 21745490
-
Expression of adiponectin and its receptors in swine.J Anim Sci. 2005 Mar;83(3):565-78. doi: 10.2527/2005.833565x. J Anim Sci. 2005. PMID: 15705753
-
Expression profiles and associations of adiponectin and adiponectin receptors with intramuscular fat in Tibetan chicken.Br Poult Sci. 2017 Apr;58(2):151-157. doi: 10.1080/00071668.2016.1268252. Epub 2017 Jan 18. Br Poult Sci. 2017. PMID: 27936897
-
Adiponectin-Resistance in Obesity.Adv Exp Med Biol. 2017;960:415-441. doi: 10.1007/978-3-319-48382-5_18. Adv Exp Med Biol. 2017. PMID: 28585210 Review.
-
Nutritional and hormonal modulation of adiponectin and its receptors adipoR1 and adipoR2.Vitam Horm. 2012;90:57-94. doi: 10.1016/B978-0-12-398313-8.00003-8. Vitam Horm. 2012. PMID: 23017712 Review.
Cited by
-
Yin Yang 1 promotes hepatic gluconeogenesis through upregulation of glucocorticoid receptor.Diabetes. 2013 Apr;62(4):1064-73. doi: 10.2337/db12-0744. Epub 2012 Nov 27. Diabetes. 2013. PMID: 23193188 Free PMC article.
-
Poor Sleep Quality Increases Gestational Weight Gain Rate in Pregnant People: Findings from the MADRES Study.Res Sq [Preprint]. 2023 Sep 25:rs.3.rs-2944456. doi: 10.21203/rs.3.rs-2944456/v1. Res Sq. 2023. PMID: 37841879 Free PMC article. Preprint.
-
Adiponectin levels among individuals with varied employment status in Japan: a cross-sectional analysis of the J-SHINE study.Sci Rep. 2019 Jul 29;9(1):10936. doi: 10.1038/s41598-019-47448-2. Sci Rep. 2019. PMID: 31358891 Free PMC article.
-
Effect of Diet and Exercise on the Peripheral Immune System in Young Balb/c Mice.Biomed Res Int. 2015;2015:458470. doi: 10.1155/2015/458470. Epub 2015 Nov 8. Biomed Res Int. 2015. PMID: 26634209 Free PMC article.
-
The Role of Diet and Interventions on Multiple Sclerosis: A Review.Nutrients. 2022 Mar 9;14(6):1150. doi: 10.3390/nu14061150. Nutrients. 2022. PMID: 35334810 Free PMC article. Review.
References
-
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

