Bidirectional metabolic regulation of neurocognitive function
- PMID: 21236352
- PMCID: PMC3084367
- DOI: 10.1016/j.nlm.2011.01.004
Bidirectional metabolic regulation of neurocognitive function
Abstract
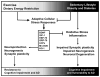
The efficiency of somatic energy metabolism is correlated with cognitive change over the lifespan. This relationship is bidirectional, with improved overall fitness associated with enhanced synaptic function and neuroprotection, and synaptic endangerment occurring in the context of impaired energy metabolism. In this review, we discuss recent advancements in the fields of exercise, dietary energy intake and diabetes, as they relate to neuronal function in the hippocampus. Because hippocampal neurons have energy requirements that are relatively higher than those of other brain regions, they are uniquely poised to benefit from exercise, and to be harmed by diabetes. We view exercise and dietary energy restriction as being associated with enhanced hippocampal plasticity at one end of a continuum, with obesity and diabetes accompanied by cognitive impairment at the other end of the continuum. Understanding the mechanisms for this continuum may yield novel therapeutic targets for the prevention and treatment of cognitive decline following aging, disease, or injury.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
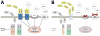
Similar articles
-
Metabolic reserve as a determinant of cognitive aging.J Alzheimers Dis. 2012;30 Suppl 2(0 2):S5-13. doi: 10.3233/JAD-2011-110899. J Alzheimers Dis. 2012. PMID: 22045480 Free PMC article. Review.
-
Impact of energy intake and expenditure on neuronal plasticity.Neuromolecular Med. 2008;10(4):209-18. doi: 10.1007/s12017-008-8043-0. Epub 2008 Jun 10. Neuromolecular Med. 2008. PMID: 18543119 Free PMC article. Review.
-
Revenge of the "sit": how lifestyle impacts neuronal and cognitive health through molecular systems that interface energy metabolism with neuronal plasticity.J Neurosci Res. 2006 Sep;84(4):699-715. doi: 10.1002/jnr.20979. J Neurosci Res. 2006. PMID: 16862541 Review.
-
Caloric restriction and brain function.Curr Opin Clin Nutr Metab Care. 2008 Nov;11(6):686-92. doi: 10.1097/MCO.0b013e328313968f. Curr Opin Clin Nutr Metab Care. 2008. PMID: 18827571 Review.
-
Opposing effects of positive and negative stress on hippocampal plasticity over the lifespan.Ageing Res Rev. 2012 Jul;11(3):399-403. doi: 10.1016/j.arr.2011.11.003. Epub 2011 Nov 10. Ageing Res Rev. 2012. PMID: 22101329 Review.
Cited by
-
Simultaneous Alteration of the Circadian Variation of Memory, Hippocampal Synaptic Plasticity, and Metabolism in a Triple Transgenic Mouse Model of Alzheimer's Disease.Front Aging Neurosci. 2022 Mar 31;14:835885. doi: 10.3389/fnagi.2022.835885. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35431906 Free PMC article.
-
Metabolic Stress Alters Antioxidant Systems, Suppresses the Adiponectin Receptor 1 and Induces Alzheimer's Like Pathology in Mice Brain.Cells. 2020 Jan 19;9(1):249. doi: 10.3390/cells9010249. Cells. 2020. PMID: 31963819 Free PMC article.
-
Carbohydrate-biased control of energy metabolism: the darker side of the selfish brain.Front Neuroenergetics. 2011 Dec 20;3:8. doi: 10.3389/fnene.2011.00008. eCollection 2011. Front Neuroenergetics. 2011. PMID: 22194720 Free PMC article. No abstract available.
-
Role of gamma-amino-butyric acid in the dorsal anterior cingulate in age-associated changes in cognition.Neuropsychopharmacology. 2018 Oct;43(11):2285-2291. doi: 10.1038/s41386-018-0134-5. Epub 2018 Jul 3. Neuropsychopharmacology. 2018. PMID: 30050047 Free PMC article.
-
Associations of Objectively-Assessed Physical Activity and Sedentary Time with Hippocampal Gray Matter Volume in Children with Overweight/Obesity.J Clin Med. 2020 Apr 10;9(4):1080. doi: 10.3390/jcm9041080. J Clin Med. 2020. PMID: 32290290 Free PMC article.
References
-
- Abbas T, Faivre E, Hölscher C. Impairment of synaptic plasticity and memory formation in GLP-1 receptor KO mice: Interaction between type 2 diabetes and Alzheimer’s disease. Behav Brain Res. 2009;205:265–7. - PubMed
-
- Anan F, Masaki T, Shimomura T, Fujiki M, Umeno Y, Eshima N, Saikawa T, Yoshimatsu H. Abdominal visceral fat accumulation is associated with hippocampus volume in non-dementia patients with type 2 diabetes mellitus. Neuroimage. 2010;49:57–62. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

