Proteolytic networks in cancer
- PMID: 21232958
- PMCID: PMC3840715
- DOI: 10.1016/j.tcb.2010.12.002
Proteolytic networks in cancer
Abstract
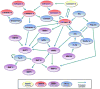
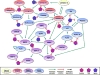
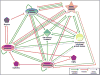
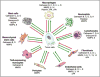
Proteases are important for multiple processes during malignant progression, including tumor angiogenesis, invasion and metastasis. Recent evidence reveals that tumor-promoting proteases function as part of an extensive multidirectional network of proteolytic interactions, in contrast to the unidirectional caspase cascade. These networks involve different constituents of the tumor microenvironment and key proteases, such as cathepsin B, urokinase-type plasminogen activator and several matrix metalloproteinases, occupy central nodes for amplifying proteolytic signals passing through the network. The proteolytic network interacts with other important signaling pathways in tumor biology, involving chemokines, cytokines, and kinases. Viewing these proteolytic interactions as a system of activating and inhibiting reactions provides insight into tumor biology and reveals relevant pharmaceutical targets. This review examines recent advances in understanding proteases in cancer and summarizes how the network of activity is co-opted to promote tumor progression.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Proteases as prognostic markers in human and canine cancers.Vet Comp Oncol. 2017 Sep;15(3):669-683. doi: 10.1111/vco.12223. Epub 2016 May 2. Vet Comp Oncol. 2017. PMID: 27136601 Review.
-
Protease signaling in tumor progression.Thromb Res. 2007;120 Suppl 2:S75-81. doi: 10.1016/S0049-3848(07)70134-2. Thromb Res. 2007. PMID: 18023718
-
Extracellular proteolysis in cancer: Proteases, substrates, and mechanisms in tumor progression and metastasis.J Biol Chem. 2024 Jun;300(6):107347. doi: 10.1016/j.jbc.2024.107347. Epub 2024 May 6. J Biol Chem. 2024. PMID: 38718867 Free PMC article. Review.
-
Proteolytic-antiproteolytic balance and its regulation in carcinogenesis.World J Gastroenterol. 2005 Mar 7;11(9):1251-66. doi: 10.3748/wjg.v11.i9.1251. World J Gastroenterol. 2005. PMID: 15761961 Free PMC article. Review.
-
Proteases and cytokines as mediators of interactions between cancer and stromal cells in tumours.Biol Chem. 2017 Jun 27;398(7):709-719. doi: 10.1515/hsz-2016-0283. Biol Chem. 2017. PMID: 28002021 Review.
Cited by
-
Macrophage biology in development, homeostasis and disease.Nature. 2013 Apr 25;496(7446):445-55. doi: 10.1038/nature12034. Nature. 2013. PMID: 23619691 Free PMC article. Review.
-
Proteolysis-a characteristic of tumor-initiating cells in murine metastatic breast cancer.Oncotarget. 2016 Sep 6;7(36):58244-58260. doi: 10.18632/oncotarget.11309. Oncotarget. 2016. PMID: 27542270 Free PMC article.
-
Enhanced lysosomal function is critical for paclitaxel resistance in cancer cells: reversed by artesunate.Acta Pharmacol Sin. 2021 Apr;42(4):624-632. doi: 10.1038/s41401-020-0445-z. Epub 2020 Jul 23. Acta Pharmacol Sin. 2021. PMID: 32704040 Free PMC article.
-
Activatable oligomerizable imaging agents for photoacoustic imaging of furin-like activity in living subjects.J Am Chem Soc. 2013 Jul 31;135(30):11015-22. doi: 10.1021/ja4010078. Epub 2013 Jul 16. J Am Chem Soc. 2013. PMID: 23859847 Free PMC article.
-
Cancer heterogeneity and metastasis: life at the edge.Clin Exp Metastasis. 2022 Feb;39(1):15-19. doi: 10.1007/s10585-021-10101-2. Epub 2021 May 17. Clin Exp Metastasis. 2022. PMID: 33999364 Review.
References
-
- Li J, Yuan J. Caspases in apoptosis and beyond. Oncogene. 2008;27(48):6194–206. - PubMed
-
- Turk B, Turk D, Salvesen GS. Regulating cysteine protease activity: essential role of protease inhibitors as guardians and regulators. Curr Pharm Des. 2002;8(18):1623–37. - PubMed
-
- Hedrich J, et al. Fetuin-A and Cystatin C Are Endogenous Inhibitors of Human Meprin Metalloproteases. Biochemistry. 2010 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

