Three-dimensional architecture of grana and stroma thylakoids of higher plants as determined by electron tomography
- PMID: 21224341
- PMCID: PMC3091084
- DOI: 10.1104/pp.110.170647
Three-dimensional architecture of grana and stroma thylakoids of higher plants as determined by electron tomography
Abstract
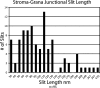
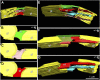
We have investigated the three-dimensional (3D) architecture of the thylakoid membranes of Arabidopsis (Arabidopsis thaliana), tobacco (Nicotiana tabacum), and spinach (Spinacia oleracea) with a resolution of approximately 7 nm by electron tomography of high-pressure-frozen/freeze-substituted intact chloroplasts. Higher-plant thylakoids are differentiated into two interconnected and functionally distinct domains, the photosystem II/light-harvesting complex II-enriched stacked grana thylakoids and the photosystem I/ATP synthase-enriched, nonstacked stroma thylakoids. The grana thylakoids are organized in the form of cylindrical stacks and are connected to the stroma thylakoids via tubular junctions. Our data confirm that the stroma thylakoids are wound around the grana stacks in the form of multiple, right-handed helices at an angle of 20° to 25° as postulated by a helical thylakoid model. The junctional connections between the grana and stroma thylakoids all have a slit-like architecture, but their size varies tremendously from approximately 15 × 30 nm to approximately 15 × 435 nm, which is approximately 5 times larger than seen in chemically fixed thylakoids. The variable slit length results in less periodicity in grana/stroma thylakoid organization than proposed in the original helical model. The stroma thylakoids also exhibit considerable architectural variability, which is dependent, in part, on the number and the orientation of adjacent grana stacks to which they are connected. Whereas some stroma thylakoids form solid, sheet-like bridges between adjacent grana, others exhibit a branching geometry with small, more tubular sheet domains also connecting adjacent, parallel stroma thylakoids. We postulate that the tremendous variability in size of the junctional slits may reflect a novel, active role of junctional slits in the regulation of photosynthetic function. In particular, by controlling the size of junctional slits, plants could regulate the flow of ions and membrane molecules between grana and stroma thylakoid membrane domains.
Figures






Similar articles
-
A brief history of how microscopic studies led to the elucidation of the 3D architecture and macromolecular organization of higher plant thylakoids.Photosynth Res. 2020 Sep;145(3):237-258. doi: 10.1007/s11120-020-00782-3. Epub 2020 Oct 5. Photosynth Res. 2020. PMID: 33017036 Free PMC article. Review.
-
Dark-adapted spinach thylakoid protein heterogeneity offers insights into the photosystem II repair cycle.Biochim Biophys Acta. 2014 Sep;1837(9):1463-71. doi: 10.1016/j.bbabio.2013.11.014. Epub 2013 Dec 1. Biochim Biophys Acta. 2014. PMID: 24296034
-
Insights into the complex 3-D architecture of thylakoid membranes in unicellular cyanobacterium Cyanothece sp. ATCC 51142.Plant Signal Behav. 2011 Apr;6(4):566-9. doi: 10.4161/psb.6.4.14946. Epub 2011 Apr 1. Plant Signal Behav. 2011. PMID: 21445014 Free PMC article.
-
Purification of structurally intact grana from plants thylakoids membranes.J Bioenerg Biomembr. 2010 Feb;42(1):37-45. doi: 10.1007/s10863-009-9261-3. Epub 2010 Jan 12. J Bioenerg Biomembr. 2010. PMID: 20066483
-
Dynamic flexibility in the structure and function of photosystem II in higher plant thylakoid membranes: the grana enigma.Photosynth Res. 2008 Oct-Dec;98(1-3):575-87. doi: 10.1007/s11120-008-9381-3. Epub 2008 Nov 8. Photosynth Res. 2008. PMID: 18998237 Review.
Cited by
-
Quality control of PSII: behavior of PSII in the highly crowded grana thylakoids under excessive light.Plant Cell Physiol. 2014 Jul;55(7):1206-15. doi: 10.1093/pcp/pcu043. Epub 2014 Mar 7. Plant Cell Physiol. 2014. PMID: 24610582 Free PMC article. Review.
-
Hydrophobic Mismatch in the Thylakoid Membrane Regulates Photosynthetic Light Harvesting.J Am Chem Soc. 2024 May 29;146(21):14905-14914. doi: 10.1021/jacs.4c05220. Epub 2024 May 17. J Am Chem Soc. 2024. PMID: 38759103 Free PMC article.
-
The quest for four-dimensional imaging in plant cell biology: it's just a matter of time.Ann Bot. 2012 Jul;110(2):461-74. doi: 10.1093/aob/mcs107. Epub 2012 May 23. Ann Bot. 2012. PMID: 22628381 Free PMC article. Review.
-
Phylogenetic Analysis of the Thylakoid ATP/ADP Carrier Reveals New Insights into Its Function Restricted to Green Plants.Front Plant Sci. 2012 Jan 9;2:110. doi: 10.3389/fpls.2011.00110. eCollection 2011. Front Plant Sci. 2012. PMID: 22629269 Free PMC article.
-
Toward understanding the multiple spatiotemporal dynamics of chlorophyll fluorescence.Plant Signal Behav. 2015;10(6):e1022014. doi: 10.1080/15592324.2015.1022014. Plant Signal Behav. 2015. PMID: 26176900 Free PMC article.
References
-
- Allen JF. (1992) Protein phosphorylation in regulation of photosynthesis. Biochim Biophys Acta 1098: 275–335 - PubMed
-
- Anderson JM. (1999) Insights into the consequences of grana stacking of thylakoid membranes in vascular plants: a personal perspective. Aust J Plant Physiol 26: 625–639
-
- Anderson JM, Andersson B. (1988) The dynamic photosynthetic membrane and regulation of solar energy conversion. Trends Biochem Sci 13: 351–355 - PubMed
-
- Anderson JM, Goodchild DJ, Boardman NK. (1973) Composition of the photosystems and chloroplast structure in extreme shade plants. Biochim Biophys Acta 325: 573–585 - PubMed
-
- Andersson B, Anderson JM. (1980) Lateral heterogeneity in the distribution of chlorophyll-protein complexes of the thylakoid membranes of spinach chloroplasts. Biochim Biophys Acta 593: 427–440 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

